Evolving Healthcare Policies
The Prophylactic HIV Drug Market is significantly influenced by evolving healthcare policies that prioritize preventive care. Recent policy shifts have emphasized the importance of integrating HIV prevention into broader healthcare frameworks, which has led to increased coverage for prophylactic treatments. For example, several countries have expanded insurance coverage for PrEP, making it more accessible to individuals at high risk of HIV exposure. This policy evolution not only encourages healthcare providers to prescribe prophylactic drugs but also fosters a supportive environment for patients seeking preventive options. As healthcare systems adapt to these changes, the Prophylactic HIV Drug Market is poised for growth, driven by enhanced patient access and a focus on preventive health measures.
Government Initiatives and Funding
Government initiatives play a pivotal role in shaping the Prophylactic HIV Drug Market. Various countries have implemented national strategies aimed at reducing HIV transmission rates, which often include funding for preventive measures. For instance, substantial investments in public health campaigns and subsidized access to PrEP have been observed, leading to increased uptake among at-risk populations. In 2023, funding for HIV prevention programs reached unprecedented levels, indicating a commitment to combating the epidemic. These initiatives not only enhance awareness but also facilitate the distribution of prophylactic drugs, thereby expanding the market. As governments continue to prioritize HIV prevention, the Prophylactic HIV Drug Market is likely to benefit from sustained financial support and policy frameworks that promote access to essential medications.
Rising Incidence of HIV Infections
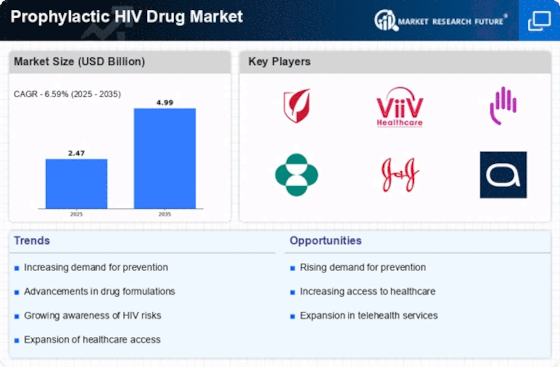
The Prophylactic HIV Drug Market is experiencing growth due to the rising incidence of HIV infections worldwide. According to recent data, approximately 1.5 million new HIV infections occur annually, underscoring the urgent need for effective preventive measures. This trend compels healthcare systems to adopt prophylactic strategies, such as pre-exposure prophylaxis (PrEP), to mitigate transmission rates. As awareness of HIV prevention increases, more individuals are seeking prophylactic options, thereby driving demand within the market. The emphasis on early intervention and prevention aligns with public health goals, further propelling the Prophylactic HIV Drug Market forward. Consequently, pharmaceutical companies are investing in research and development to create innovative prophylactic solutions that cater to diverse populations, enhancing accessibility and adherence.
Increased Focus on At-Risk Populations
The Prophylactic HIV Drug Market is witnessing a heightened focus on at-risk populations, which is driving demand for preventive solutions. Targeted outreach programs aimed at communities disproportionately affected by HIV, such as men who have sex with men and intravenous drug users, are gaining momentum. These initiatives often include education on the benefits of prophylactic treatments, leading to increased awareness and uptake of PrEP. Data suggests that tailored interventions can significantly reduce new infections within these populations, reinforcing the importance of targeted strategies. As public health efforts continue to prioritize these groups, the Prophylactic HIV Drug Market is likely to expand, with a growing emphasis on accessibility and culturally competent care.
Technological Innovations in Drug Delivery
Technological innovations are transforming the Prophylactic HIV Drug Market by enhancing drug delivery methods. Advances in formulation science and delivery systems, such as long-acting injectables and implantable devices, are emerging as viable alternatives to daily oral PrEP. These innovations aim to improve adherence rates among users, addressing a critical barrier in HIV prevention. For instance, recent studies indicate that long-acting injectable formulations can provide protection for up to three months, significantly reducing the burden of daily medication. As these technologies gain traction, they are likely to attract investment and interest from both healthcare providers and patients. The Prophylactic HIV Drug Market stands to benefit from these advancements, as they offer new avenues for effective HIV prevention.