Growing Regulatory Pressure
The Pharmaceutical Intermediate Market is facing increasing regulatory scrutiny, which is shaping the landscape of pharmaceutical manufacturing. Regulatory bodies are enforcing stringent guidelines to ensure the quality and safety of pharmaceutical products. In 2025, it is anticipated that compliance costs for pharmaceutical manufacturers will rise, potentially reaching USD 50 billion. This regulatory pressure compels companies to invest in high-quality intermediates that meet the required standards. As a result, manufacturers may prioritize sourcing intermediates from reputable suppliers, thereby influencing market dynamics. The need for compliance with Good Manufacturing Practices (GMP) and other regulations is likely to drive innovation in the production processes of pharmaceutical intermediates, ensuring that they align with the evolving regulatory framework.
Increased Investment in R&D
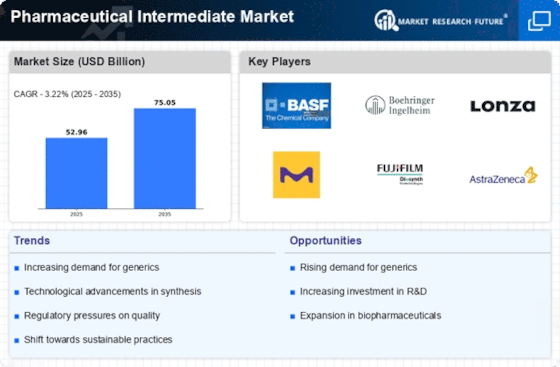
Investment in research and development (R&D) within the Pharmaceutical Intermediate Market is on the rise, as companies strive to innovate and develop new therapeutic agents. The pharmaceutical sector is increasingly focusing on personalized medicine and biologics, which require specialized intermediates for their production. In 2025, R&D spending in the pharmaceutical sector is expected to exceed USD 200 billion, reflecting a commitment to advancing drug discovery and development. This heightened investment is likely to drive demand for pharmaceutical intermediates, as they play a crucial role in the synthesis of complex molecules. Furthermore, the emphasis on developing novel drug formulations may lead to the emergence of new intermediates, thereby expanding the market landscape.
Expansion of Biopharmaceuticals
The Pharmaceutical Intermediate Market is witnessing a significant expansion in the biopharmaceutical sector, which is reshaping the demand for intermediates. Biopharmaceuticals, including monoclonal antibodies and recombinant proteins, require specialized intermediates for their production. The biopharmaceutical market is projected to grow at a compound annual growth rate (CAGR) of over 10% through 2025, indicating a robust demand for pharmaceutical intermediates tailored for these applications. This growth is likely to encourage manufacturers to develop innovative intermediates that cater to the specific needs of biopharmaceutical production. As the industry evolves, the integration of advanced technologies in the synthesis of intermediates may further enhance efficiency and yield, thereby supporting the burgeoning biopharmaceutical market.
Rising Demand for Generic Drugs
The Pharmaceutical Intermediate Market is experiencing a notable increase in demand for generic drugs. This trend is primarily driven by the rising healthcare costs and the need for affordable medication options. As patents for several blockbuster drugs expire, generic alternatives are becoming more accessible, leading to a surge in the production of pharmaceutical intermediates. In 2025, the market for generic drugs is projected to reach approximately USD 400 billion, indicating a robust growth trajectory. This demand for generics necessitates a corresponding increase in the supply of pharmaceutical intermediates, which are essential for the synthesis of these drugs. Consequently, manufacturers are likely to invest in the development of efficient processes to produce high-quality intermediates, thereby enhancing their market position.
Emerging Markets and Globalization
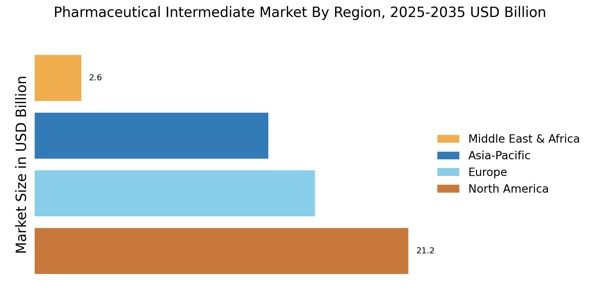
The Pharmaceutical Intermediate Market is increasingly influenced by the dynamics of emerging markets and globalization. Countries with developing economies are witnessing a surge in pharmaceutical manufacturing capabilities, driven by lower production costs and favorable regulatory environments. In 2025, it is estimated that the pharmaceutical market in emerging economies will account for nearly 30% of the total market share. This shift is likely to create new opportunities for pharmaceutical intermediates, as manufacturers in these regions seek to establish a competitive edge. Additionally, globalization is facilitating the cross-border trade of intermediates, allowing companies to source materials from diverse locations. This interconnectedness may lead to increased competition and innovation within the pharmaceutical intermediate sector, ultimately benefiting the industry as a whole.