Rising Healthcare Expenditure
Healthcare expenditure in the US is on the rise, which positively impacts the montelukast intermediate market. As healthcare spending increases, there is a corresponding growth in the demand for effective medications, including montelukast. The US healthcare expenditure is expected to reach $6 trillion by 2027, reflecting a significant investment in health services and pharmaceuticals. This trend suggests that patients are more likely to seek treatment for respiratory conditions, thereby driving the demand for montelukast and its intermediates. Furthermore, increased insurance coverage and access to healthcare services may lead to higher prescription rates, further bolstering the montelukast intermediate market.
Focus on Chronic Disease Management
The montelukast intermediate market is influenced by the growing focus on chronic disease management in the US. As healthcare providers emphasize the importance of managing chronic conditions such as asthma, the demand for effective treatments like montelukast is likely to increase. The National Heart, Lung, and Blood Institute indicates that asthma affects millions of Americans, necessitating ongoing treatment strategies. This focus on chronic disease management may lead to increased prescriptions of montelukast, thereby driving the montelukast intermediate market. Additionally, healthcare policies that promote preventive care and chronic disease management could further enhance the market's growth prospects.
Expansion of Pharmaceutical Manufacturing
The montelukast intermediate market is benefiting from the expansion of pharmaceutical manufacturing capabilities in the US. With a focus on increasing production efficiency and meeting the growing demand for montelukast, manufacturers are investing in advanced production technologies. The US pharmaceutical industry is projected to reach $600 billion by 2025, indicating a robust environment for the montelukast intermediate market. This expansion is likely to enhance the availability of montelukast intermediates, thereby supporting the overall growth of the market. Additionally, the trend towards outsourcing production to specialized facilities may further streamline the supply chain, ensuring that the montelukast intermediate market remains competitive and responsive to market needs.
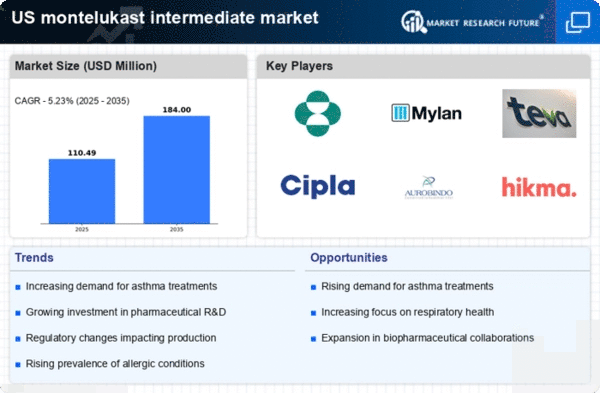
Increasing Prevalence of Allergic Conditions
The montelukast intermediate market is experiencing growth due to the rising prevalence of allergic conditions such as asthma and allergic rhinitis in the US. According to the CDC, approximately 8.4% of children and 7.7% of adults are diagnosed with asthma, leading to a heightened demand for effective treatments. Montelukast, as a leukotriene receptor antagonist, plays a crucial role in managing these conditions. The increasing awareness of asthma management and the need for preventive therapies are likely to drive the montelukast intermediate market. Furthermore, the growing population and urbanization contribute to higher exposure to allergens, which may further escalate the demand for montelukast and its intermediates in the pharmaceutical industry.
Emerging Research and Development Initiatives
The montelukast intermediate market is poised for growth due to emerging research and development initiatives aimed at enhancing the efficacy and safety of montelukast. Pharmaceutical companies in the US are increasingly investing in R&D to explore new formulations and delivery methods for montelukast. This focus on innovation is likely to lead to the development of improved products that cater to specific patient needs, thereby expanding the market. Furthermore, collaborations between academic institutions and industry players may foster advancements in drug development, potentially leading to new applications for montelukast. Such initiatives could significantly impact the montelukast intermediate market, driving demand for intermediates used in the production of these innovative therapies.