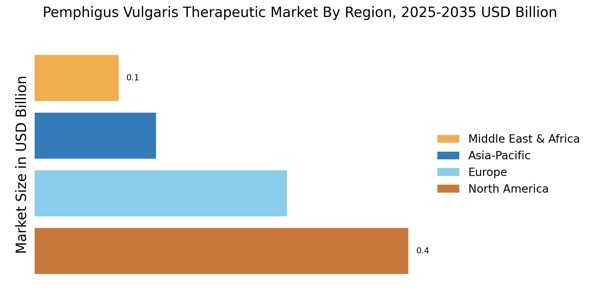Advancements in Treatment Modalities
Recent advancements in treatment modalities are significantly influencing the Pemphigus Vulgaris Therapeutic Market. The introduction of novel biologic therapies, such as monoclonal antibodies, has transformed the therapeutic landscape for pemphigus vulgaris. These innovative treatments offer targeted mechanisms of action, potentially leading to improved patient outcomes. Furthermore, the market has witnessed the emergence of combination therapies that enhance efficacy while minimizing side effects. As these advancements continue to evolve, they are likely to attract investment and research focus, thereby driving market growth. The Pemphigus Vulgaris Therapeutic Market is expected to benefit from ongoing clinical trials and research initiatives aimed at optimizing treatment protocols and improving patient quality of life.
Rising Awareness and Patient Advocacy
The rising awareness of pemphigus vulgaris and the role of patient advocacy groups are vital drivers for the Pemphigus Vulgaris Therapeutic Market. Increased awareness campaigns have led to better recognition of the disease among healthcare professionals and the general public. Patient advocacy organizations are actively working to educate patients about their condition and available treatment options, which may lead to earlier diagnosis and treatment initiation. This heightened awareness is likely to result in a larger patient population seeking therapeutic interventions, thereby stimulating market demand. The Pemphigus Vulgaris Therapeutic Market stands to benefit from these advocacy efforts, as they promote research funding and support for innovative therapies.
Increasing Prevalence of Pemphigus Vulgaris
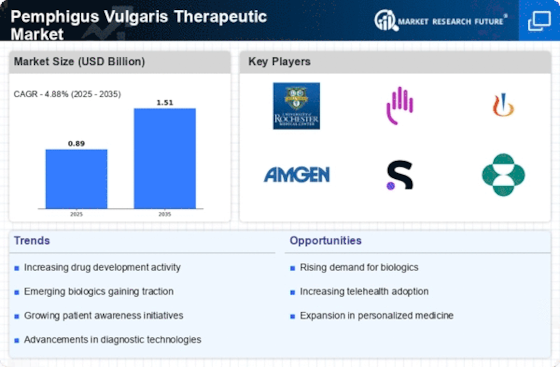
The rising incidence of pemphigus vulgaris is a pivotal driver for the Pemphigus Vulgaris Therapeutic Market. Recent estimates suggest that the prevalence of this autoimmune disorder is approximately 0.5 to 3 cases per 100,000 individuals. This increasing prevalence necessitates the development of effective therapeutic options, thereby propelling market growth. As awareness of pemphigus vulgaris expands, more patients are likely to seek treatment, further stimulating demand for innovative therapies. The growing patient population is expected to create a robust market environment, encouraging pharmaceutical companies to invest in research and development. Consequently, the Pemphigus Vulgaris Therapeutic Market is poised for expansion as healthcare providers strive to meet the needs of an increasing number of patients.
Regulatory Support and Fast-Track Approvals
Regulatory support for new treatments is a significant driver of the Pemphigus Vulgaris Therapeutic Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for therapies targeting rare diseases, including pemphigus vulgaris. Initiatives such as orphan drug designations and fast-track approvals are facilitating quicker access to innovative treatments for patients. This supportive regulatory environment encourages pharmaceutical companies to invest in the development of new therapies, knowing that they may benefit from streamlined approval processes. As a result, the Pemphigus Vulgaris Therapeutic Market is likely to see a surge in new product launches, enhancing treatment options for patients and driving market growth.
Growing Investment in Research and Development
The increasing investment in research and development is a crucial driver for the Pemphigus Vulgaris Therapeutic Market. Pharmaceutical companies are allocating substantial resources to explore new therapeutic options and improve existing treatments. This trend is underscored by the rising number of clinical trials focused on pemphigus vulgaris, which have surged in recent years. According to recent data, the number of clinical trials has increased by over 30% in the past five years, reflecting a heightened interest in this therapeutic area. Such investments not only foster innovation but also enhance the competitive landscape, as companies strive to bring novel therapies to market. The Pemphigus Vulgaris Therapeutic Market is likely to experience accelerated growth as a result of these research initiatives.