Investment in Research and Development
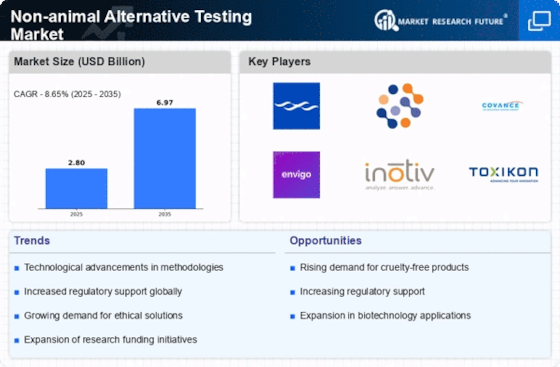
Investment in research and development is a critical driver for the Non-animal Alternative Testing Market. Increased funding from both public and private sectors is enabling the exploration of innovative testing methodologies that do not rely on animal subjects. Reports indicate that R&D spending in this sector is projected to exceed USD 1 billion by 2027, highlighting the commitment to advancing non-animal testing technologies. This influx of capital is likely to accelerate the pace of innovation, leading to the introduction of more effective and reliable testing solutions that align with ethical standards.
Regulatory Support for Non-animal Testing
Regulatory bodies are increasingly endorsing non-animal testing methods, which significantly influences the Non-animal Alternative Testing Market. Initiatives such as the European Union's REACH regulation and the U.S. FDA's commitment to alternative methods are paving the way for broader acceptance of these testing approaches. This regulatory support not only encourages companies to adopt non-animal methods but also fosters innovation in the sector. As of 2025, it is estimated that over 50% of new drug applications will utilize non-animal testing data, reflecting a paradigm shift in regulatory expectations and practices.
Collaboration Between Industry and Academia
Collaboration between industry stakeholders and academic institutions is fostering innovation within the Non-animal Alternative Testing Market. These partnerships facilitate the exchange of knowledge and resources, leading to the development of novel testing methods and technologies. For example, joint research initiatives have resulted in the creation of advanced in vitro models that better mimic human biology. Such collaborations are essential for validating new testing methods, which can enhance their acceptance in regulatory frameworks. As these partnerships continue to grow, they are expected to play a crucial role in advancing the non-animal testing landscape.
Consumer Awareness and Demand for Ethical Testing
There is a growing consumer awareness regarding ethical testing practices, which is driving the Non-animal Alternative Testing Market. Consumers are increasingly advocating for cruelty-free products, prompting companies to seek alternatives to animal testing. This shift in consumer sentiment is reflected in market trends, with a reported 70% of consumers willing to pay more for products that are not tested on animals. As brands respond to this demand, the adoption of non-animal testing methods is likely to accelerate, further solidifying the market's growth trajectory.
Technological Advancements in Non-animal Alternative Testing
The Non-animal Alternative Testing Market is experiencing a surge in technological advancements that enhance the efficacy and reliability of testing methods. Innovations such as in vitro testing, organ-on-a-chip technologies, and computational modeling are becoming increasingly prevalent. These technologies not only reduce the reliance on animal testing but also provide more accurate and human-relevant data. For instance, the market for in vitro testing is projected to reach USD 5 billion by 2026, indicating a robust growth trajectory. As these technologies evolve, they are likely to attract more investment and research, further propelling the Non-animal Alternative Testing Market forward.