Rising Prevalence of HIV
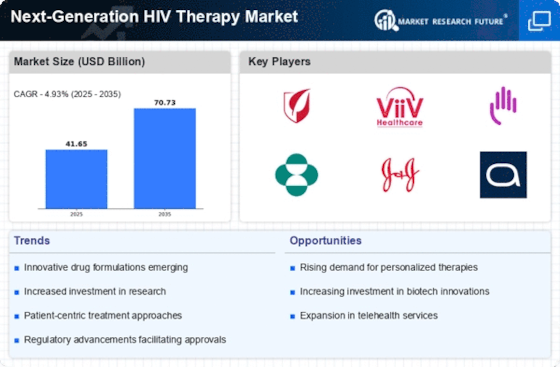
The increasing prevalence of HIV infections worldwide is a primary driver for the Next-Generation HIV Therapy Market. As of recent estimates, approximately 38 million people are living with HIV, with a significant number unaware of their status. This growing population necessitates innovative treatment options that are more effective and have fewer side effects. The demand for next-generation therapies is further fueled by the need for improved adherence to treatment regimens, as traditional therapies often require complex dosing schedules. Consequently, pharmaceutical companies are investing heavily in research and development to create therapies that not only enhance patient outcomes but also address the challenges posed by drug resistance. This trend indicates a robust market potential for next-generation therapies that can effectively manage HIV.
Advancements in Biotechnology
Technological advancements in biotechnology are propelling the Next-Generation HIV Therapy Market forward. Innovations such as CRISPR gene editing and monoclonal antibodies are paving the way for novel therapeutic approaches. These biotechnological breakthroughs enable the development of therapies that can target the virus more precisely, potentially leading to functional cures. The market is witnessing a surge in clinical trials focusing on these advanced therapies, with several candidates showing promising results in early-stage studies. For instance, therapies that utilize long-acting injectables are gaining traction, as they offer the possibility of reducing the frequency of dosing while maintaining viral suppression. This shift towards biotechnological solutions is likely to reshape the landscape of HIV treatment, making it more effective and patient-friendly.
Growing Awareness and Education
Growing awareness and education about HIV and its treatment options are driving the Next-Generation HIV Therapy Market. Public health campaigns and educational initiatives have significantly improved knowledge about HIV transmission, prevention, and treatment. As individuals become more informed about their health, there is a corresponding increase in demand for advanced therapies that offer better efficacy and convenience. This heightened awareness is particularly evident among high-risk populations, who are actively seeking out the latest treatment options. Additionally, healthcare providers are increasingly emphasizing the importance of personalized treatment plans, which align with the principles of next-generation therapies. This trend suggests that as awareness continues to rise, the market for innovative HIV therapies will likely expand, catering to the evolving needs of patients.
Increased Funding for HIV Research
The Next-Generation HIV Therapy Market is significantly influenced by increased funding for HIV research and development. Governments and non-profit organizations are allocating substantial resources to combat the HIV epidemic, recognizing the need for innovative solutions. In recent years, funding for HIV-related research has seen a notable rise, with billions of dollars directed towards developing new therapies and improving existing ones. This financial support is crucial for fostering innovation and accelerating the development of next-generation therapies. Moreover, partnerships between public and private sectors are becoming more common, facilitating the sharing of knowledge and resources. Such collaborations are expected to enhance the speed and efficiency of bringing new therapies to market, ultimately benefiting patients and healthcare systems alike.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a crucial driver of the Next-Generation HIV Therapy Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for breakthrough therapies that address unmet medical needs. Initiatives such as the FDA's Fast Track designation and Breakthrough Therapy designation are designed to facilitate the development and approval of promising new treatments. This supportive regulatory environment encourages pharmaceutical companies to invest in research and development, knowing that their innovative therapies may receive faster market access. As a result, the pipeline for next-generation HIV therapies is becoming more robust, with numerous candidates in various stages of development. This trend indicates a favorable outlook for the market, as regulatory support is likely to enhance the availability of effective treatment options for individuals living with HIV.