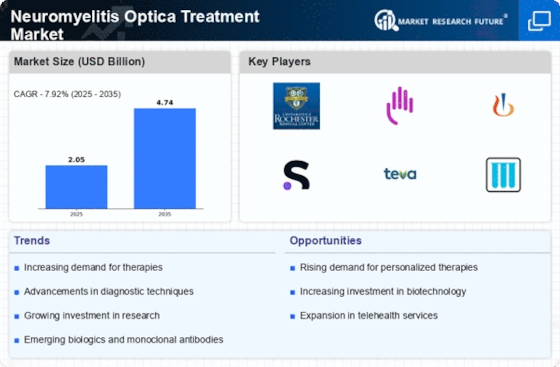
Increased Research Funding
The Neuromyelitis Optica Treatment Market is benefiting from a notable increase in research funding aimed at understanding and treating NMO. Government agencies and private organizations are allocating substantial resources to investigate the pathophysiology of NMO and to develop novel therapeutic strategies. This influx of funding is fostering collaboration between academic institutions, pharmaceutical companies, and healthcare providers, leading to accelerated research initiatives. As a result, the pace of clinical trials is likely to increase, bringing new treatments to market more rapidly. Furthermore, this heightened focus on research may lead to the discovery of biomarkers for early diagnosis and personalized treatment approaches, ultimately enhancing patient outcomes. The commitment to advancing NMO research is a critical driver for the Neuromyelitis Optica Treatment Market, as it lays the groundwork for future innovations and improved care.
Growing Awareness and Education
The growing awareness and education surrounding Neuromyelitis Optica are crucial drivers for the Neuromyelitis Optica Treatment Market. Increased efforts by healthcare organizations and advocacy groups to educate both patients and healthcare professionals about NMO are leading to earlier diagnosis and treatment. As awareness campaigns proliferate, more individuals are likely to seek medical attention for symptoms associated with NMO, resulting in a larger patient population requiring treatment. This heightened awareness not only facilitates timely intervention but also encourages healthcare providers to stay informed about the latest treatment options available. Consequently, the demand for effective therapies is expected to rise, propelling the Neuromyelitis Optica Treatment Market forward. Additionally, as patients become more informed about their condition, they may actively participate in treatment decisions, further driving the market.
Advancements in Therapeutic Options
The Neuromyelitis Optica Treatment Market is experiencing a surge in therapeutic advancements, particularly with the introduction of monoclonal antibodies and other targeted therapies. These innovative treatments, such as eculizumab and inebilizumab, have shown promising results in clinical trials, demonstrating their efficacy in reducing relapse rates and improving patient outcomes. The approval of these therapies has significantly altered the treatment paradigm for NMO, providing patients with more effective options than traditional immunosuppressants. As the understanding of the underlying mechanisms of NMO deepens, further advancements in therapeutic options are anticipated, potentially leading to the development of even more effective treatments. This evolution in therapy not only enhances patient care but also stimulates growth within the Neuromyelitis Optica Treatment Market, as healthcare providers seek to adopt the latest evidence-based practices.
Rising Prevalence of Neuromyelitis Optica
The increasing incidence of Neuromyelitis Optica (NMO) is a pivotal driver for the Neuromyelitis Optica Treatment Market. Recent studies indicate that the prevalence of NMO is on the rise, with estimates suggesting that it affects approximately 4 to 10 individuals per 100,000 people. This growing patient population necessitates the development and availability of effective treatment options. As awareness of NMO expands among healthcare professionals and patients alike, the demand for specialized therapies is likely to increase. Consequently, pharmaceutical companies are investing in research and development to create innovative treatments tailored to the unique needs of NMO patients. This trend not only enhances the treatment landscape but also propels the Neuromyelitis Optica Treatment Market forward, as stakeholders seek to address the unmet medical needs of this population.
Regulatory Support for Innovative Treatments
Regulatory bodies are increasingly providing support for the development of innovative treatments within the Neuromyelitis Optica Treatment Market. Initiatives aimed at expediting the approval process for new therapies are encouraging pharmaceutical companies to invest in research and development. This regulatory environment fosters innovation, allowing for the rapid introduction of novel treatments that address the specific needs of NMO patients. Furthermore, the establishment of orphan drug designations for certain therapies enhances the commercial viability of developing treatments for rare diseases like NMO. As a result, the Neuromyelitis Optica Treatment Market is likely to witness an influx of new products, expanding the range of options available to patients and healthcare providers. This supportive regulatory landscape not only stimulates market growth but also enhances the overall treatment experience for individuals affected by NMO.