Regulatory Support for Drug Approvals
Regulatory support for the approval of new drugs is a vital driver of the Neurological Disorder Drug Market. Regulatory agencies are increasingly recognizing the urgent need for effective treatments for neurological disorders and are streamlining the approval processes for innovative therapies. Initiatives such as accelerated approval pathways and orphan drug designations are designed to expedite the availability of new medications for conditions with limited treatment options. This supportive regulatory environment encourages pharmaceutical companies to invest in research and development, knowing that their products may reach the market more swiftly. As a result, the Neurological Disorder Drug Market is likely to experience growth as new therapies become available to patients in need.
Growing Investment in Neurological Research
The increasing investment in neurological research is a significant driver of the Neurological Disorder Drug Market. Governments and private organizations are allocating substantial funds to understand the underlying mechanisms of neurological disorders and to develop new therapeutic options. In recent years, funding for neuroscience research has surged, with billions of dollars directed towards initiatives aimed at discovering innovative treatments. This influx of capital not only supports basic research but also fosters collaboration between academic institutions and pharmaceutical companies, leading to the accelerated development of new drugs. As research continues to advance, the market is likely to benefit from a pipeline of promising therapies targeting various neurological conditions.
Advancements in Drug Development Technologies
Technological advancements in drug development are transforming the Neurological Disorder Drug Market. Innovations such as artificial intelligence, machine learning, and high-throughput screening are enhancing the efficiency and accuracy of drug discovery processes. These technologies enable researchers to identify potential drug candidates more rapidly, reducing the time and cost associated with bringing new treatments to market. For instance, the integration of AI in clinical trials has shown promise in optimizing patient selection and improving trial outcomes. As these technologies continue to evolve, they are expected to facilitate the development of novel therapies for neurological disorders, thereby expanding the market and addressing unmet medical needs.
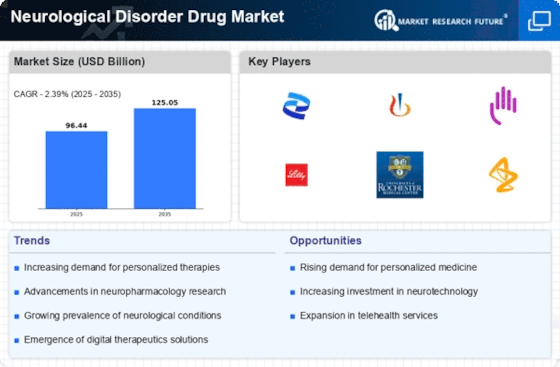
Increasing Prevalence of Neurological Disorders
The rising incidence of neurological disorders, such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis, is a primary driver of the Neurological Disorder Drug Market. According to recent estimates, the number of individuals affected by these conditions is projected to increase significantly, with Alzheimer's alone expected to affect over 14 million people by 2050. This growing patient population necessitates the development and availability of effective pharmacological treatments, thereby propelling the market forward. As healthcare systems grapple with the implications of this increase, the demand for innovative therapies and drugs tailored to specific neurological conditions is likely to intensify, further stimulating growth within the Neurological Disorder Drug Market.
Aging Population and Its Impact on Neurological Disorders
The aging population is a critical factor influencing the Neurological Disorder Drug Market. As individuals age, the risk of developing neurological disorders increases, leading to a higher demand for effective treatments. The demographic shift towards an older population is evident, with projections indicating that by 2030, one in six people will be aged 60 or over. This demographic trend is expected to drive the prevalence of age-related neurological conditions, thereby creating a substantial market for drugs aimed at managing these disorders. Pharmaceutical companies are likely to focus on developing therapies that cater specifically to the needs of older adults, further propelling growth in the Neurological Disorder Drug Market.