Expansion of Biopharmaceuticals
The expansion of biopharmaceuticals in Italy is significantly impacting the pharmacovigilance market. As the biopharmaceutical sector continues to grow, the complexity of monitoring the safety of these products increases. Biologics often have unique safety profiles, necessitating specialized pharmacovigilance strategies. This has led to a surge in demand for tailored pharmacovigilance services that can address the specific challenges associated with biopharmaceuticals. The market for pharmacovigilance services related to biopharmaceuticals is projected to reach €300 million by 2027, indicating a robust growth trajectory. Consequently, companies are investing in specialized training and resources to enhance their pharmacovigilance capabilities in this area.
Growing Focus on Patient Safety
In Italy, there is an increasing focus on patient safety, which is significantly influencing the pharmacovigilance market. Healthcare stakeholders, including regulatory authorities and healthcare providers, are prioritizing the monitoring of adverse drug reactions (ADRs) to ensure the safety of medications. This growing emphasis on patient safety is prompting pharmaceutical companies to enhance their pharmacovigilance practices. The market is expected to witness a surge in demand for advanced data analytics and reporting tools, which are essential for effective ADR monitoring. As patient safety becomes a central concern, the pharmacovigilance market is likely to expand, with investments in innovative technologies projected to reach €500 million by 2026.
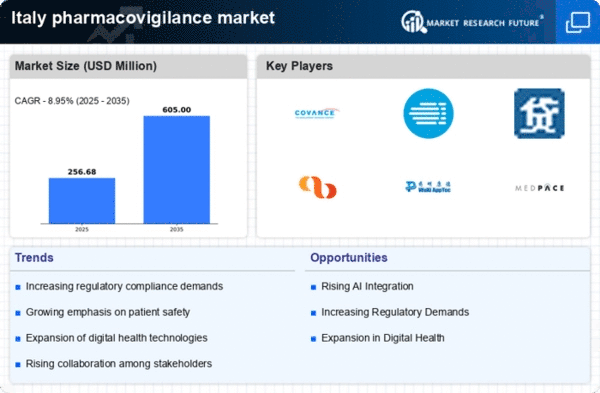
Rising Regulatory Compliance Demands
The pharmacovigilance market in Italy is experiencing heightened demands for regulatory compliance. Regulatory bodies, such as the Italian Medicines Agency (AIFA), are enforcing stricter guidelines for drug safety monitoring. This has led to an increased need for robust pharmacovigilance systems to ensure compliance with national and European regulations. As a result, pharmaceutical companies are investing significantly in pharmacovigilance solutions, with the market projected to grow at a CAGR of approximately 8% over the next five years. The emphasis on compliance not only enhances patient safety but also mitigates the risk of costly penalties for non-compliance, thereby driving the growth of the pharmacovigilance market in Italy.
Rising Public Awareness of Drug Safety
Public awareness regarding drug safety is on the rise in Italy, which is influencing the pharmacovigilance market. Patients are becoming more informed about the potential risks associated with medications, leading to increased reporting of adverse drug reactions. This heightened awareness is prompting pharmaceutical companies to strengthen their pharmacovigilance efforts to maintain public trust and ensure compliance with safety regulations. As a result, there is a growing demand for transparent communication strategies and effective risk management plans. The pharmacovigilance market is likely to benefit from this trend, as companies invest in initiatives aimed at enhancing patient engagement and safety monitoring.
Increased Adoption of Digital Solutions
The adoption of digital solutions is transforming the pharmacovigilance market in Italy. With the rise of electronic health records (EHRs) and mobile health applications, there is a growing need for integrated pharmacovigilance systems that can efficiently collect and analyze data on drug safety. This trend is driven by the desire for real-time monitoring and reporting of adverse events. Pharmaceutical companies are increasingly leveraging artificial intelligence (AI) and machine learning (ML) to enhance their pharmacovigilance capabilities. The market for digital pharmacovigilance solutions is anticipated to grow by 15% annually, reflecting the industry's shift towards more efficient and effective safety monitoring practices.