Market Growth Projections
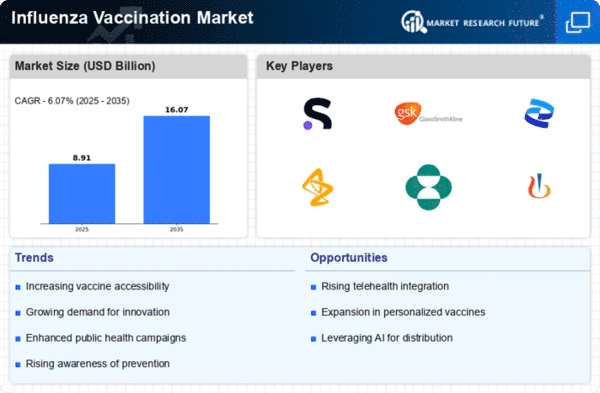
The Global Influenza Vaccines Market Industry is projected to experience robust growth, with estimates indicating a market value of 8.4 USD Billion in 2024 and a potential increase to 17.0 USD Billion by 2035. This growth trajectory suggests a compound annual growth rate of 6.64% from 2025 to 2035. Such projections highlight the increasing demand for influenza vaccines driven by various factors, including rising awareness, government initiatives, and technological advancements. The market's expansion reflects a global commitment to improving public health and preventing influenza outbreaks.
Rising Incidence of Influenza
The increasing incidence of influenza globally drives the Global Influenza Vaccines Market Industry. According to health authorities, seasonal influenza affects millions of people each year, leading to significant morbidity and mortality. In 2024, the market is projected to reach 8.4 USD Billion, reflecting the urgent need for effective vaccination strategies. Countries are enhancing their vaccination programs to mitigate the impact of influenza outbreaks, which can strain healthcare systems. This trend is likely to continue as public health initiatives emphasize the importance of vaccination in preventing influenza-related complications.
Public Awareness and Education
Public awareness and education regarding the benefits of influenza vaccination significantly influence the Global Influenza Vaccines Market Industry. Health organizations are actively promoting the importance of vaccination through various channels, including social media, community outreach, and educational programs. This increased awareness helps to dispel myths and misconceptions about vaccines, encouraging more individuals to get vaccinated. As vaccination rates rise, the demand for influenza vaccines is expected to grow, contributing to the overall market expansion. The ongoing efforts to educate the public are likely to have a lasting impact on vaccination behaviors.
Emerging Markets and Globalization
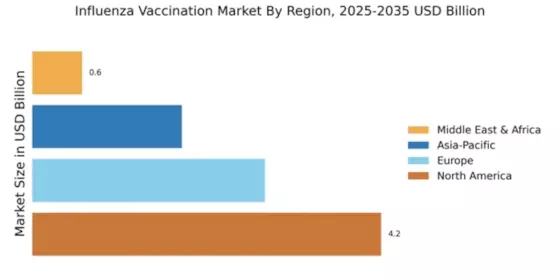
Emerging markets and globalization are pivotal drivers of the Global Influenza Vaccines Market Industry. As economies develop, there is a growing recognition of the importance of vaccination in public health. Countries in Asia, Africa, and Latin America are increasingly investing in healthcare infrastructure, including vaccine distribution systems. This trend is fostering greater access to influenza vaccines in regions that previously faced challenges in vaccination coverage. The expansion of global supply chains also facilitates the distribution of vaccines, making them more accessible to diverse populations. Consequently, the market is poised for substantial growth in these regions.
Government Initiatives and Funding
Government initiatives and funding play a crucial role in the growth of the Global Influenza Vaccines Market Industry. Many countries are increasing their investments in vaccine research and development, aiming to improve vaccine efficacy and accessibility. For instance, various national health departments have launched campaigns to promote vaccination and allocate budgets for purchasing vaccines. This financial support is essential for ensuring that vaccines are available to vulnerable populations, thereby increasing vaccination rates. As a result, the market is expected to grow significantly, with projections indicating a rise to 17.0 USD Billion by 2035.
Technological Advancements in Vaccine Development
Technological advancements in vaccine development are transforming the Global Influenza Vaccines Market Industry. Innovations such as mRNA technology and recombinant vaccines are enhancing the effectiveness and speed of vaccine production. These advancements allow for rapid responses to emerging influenza strains, which is critical in a globalized world where viruses can spread quickly. The ability to produce vaccines more efficiently may lead to increased vaccination rates and better public health outcomes. As the industry adapts to these technologies, it is likely to experience a compound annual growth rate of 6.64% from 2025 to 2035.