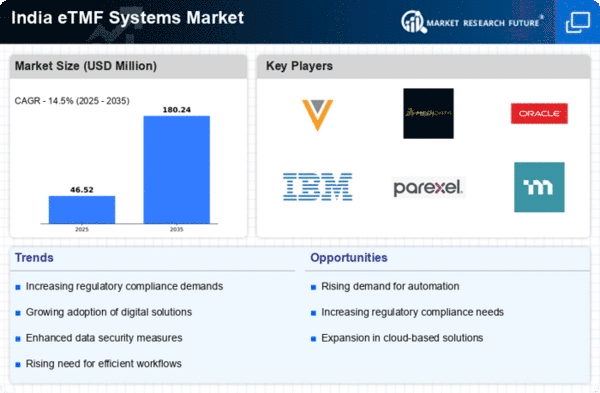
Regulatory Compliance and Quality Assurance
In the context of the etmf systems market, regulatory compliance remains a critical driver for adoption in India. The pharmaceutical industry is subject to stringent regulations from authorities such as the Central Drugs Standard Control Organization (CDSCO). As companies strive to meet these regulatory requirements, the demand for etmf systems that ensure data integrity and traceability is likely to increase. The ability to maintain comprehensive audit trails and facilitate inspections is paramount. Furthermore, organizations that utilize etmf systems can potentially reduce the risk of non-compliance, which can lead to costly penalties. This focus on quality assurance and adherence to regulatory standards is expected to propel the etmf systems market forward, as companies seek to enhance their operational frameworks.
Increased Focus on Patient-Centric Approaches
The eTMF systems market is also influenced by the growing emphasis on patient-centric approaches in clinical research. Organizations are increasingly prioritizing patient engagement and experience, which necessitates the collection and management of diverse data types. This shift towards patient-centricity requires etmf systems that can accommodate various data sources, including patient-reported outcomes and real-world evidence. As the demand for such capabilities rises, the etmf systems market is likely to expand. Companies that can offer solutions that enhance patient engagement and streamline data collection processes may find themselves at a competitive advantage. This trend reflects a broader movement within the healthcare sector towards more personalized and responsive research methodologies.
Technological Advancements in Data Management
Technological advancements are playing a pivotal role in shaping the etmf systems market in India. Innovations such as artificial intelligence (AI) and machine learning (ML) are being integrated into etmf solutions, enhancing data management capabilities. These technologies enable organizations to automate data entry, improve data accuracy, and facilitate real-time analytics. As companies increasingly adopt these advanced technologies, the etmf systems market is expected to witness substantial growth. The integration of AI and ML can lead to improved decision-making processes and more efficient trial management. Furthermore, the potential for predictive analytics to identify trends and optimize trial designs may further drive the adoption of etmf systems in the Indian market.
Rising Demand for Efficient Document Management
The etmf systems market in India is experiencing a notable surge in demand for efficient document management solutions. Organizations are increasingly recognizing the need to streamline their clinical trial processes, which has led to a growing interest in electronic trial master file systems. This shift is driven by the necessity to enhance operational efficiency and reduce time-to-market for new drugs. According to recent estimates, The market for document management solutions in India is projected to grow at a CAGR of approximately 15% from 2024 to 2029. This growth is indicative of the broader trend towards digitization in the pharmaceutical and clinical research sectors, where the etmf systems market plays a crucial role in facilitating compliance and improving data accessibility.
Growth of Clinical Trials and Research Activities
The eTMF systems market is poised for growth due to the increasing number of clinical trials and research activities in India. The country has emerged as a hub for clinical research, driven by a large patient population and a diverse genetic pool. As of 2025, the number of clinical trials registered in India has seen a significant uptick, with estimates suggesting a growth rate of around 20% annually. This expansion necessitates robust etmf systems to manage the vast amounts of data generated during trials. The ability to efficiently organize, store, and retrieve trial data is essential for researchers and sponsors alike. Consequently, the etmf systems market is likely to benefit from this trend, as organizations seek to implement solutions that can support their growing research needs.