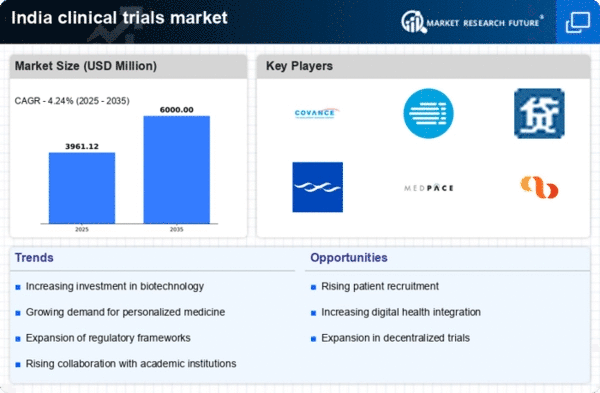
Expansion of Clinical Trial Networks
The expansion of clinical trial networks across India is a pivotal driver for the clinical trials market. With the establishment of numerous research institutions and collaborations between hospitals and pharmaceutical companies, the accessibility of clinical trials is improving. In 2025, it is estimated that the number of active clinical trial sites in India will increase by 20%, facilitating a broader reach for patient recruitment. This expansion not only enhances the diversity of patient populations but also accelerates the timeline for trial completion. As a result, the clinical trials market is poised for growth, as more sponsors are likely to consider India as a viable location for conducting trials due to its vast patient pool and cost-effective solutions.
Growing Demand for Personalized Medicine
The shift towards personalized medicine is significantly influencing the clinical trials market in India. As healthcare becomes more tailored to individual patient needs, there is an increasing requirement for clinical trials that focus on specific genetic profiles and biomarkers. This trend is reflected in the rising number of trials aimed at developing targeted therapies, which are projected to account for over 30% of all clinical trials by 2026. The clinical trials market is adapting to this demand by incorporating advanced technologies such as genomics and bioinformatics, which are essential for the successful implementation of personalized medicine. Consequently, this focus on individualized treatment options is likely to drive growth and innovation within the clinical trials market.
Regulatory Support for Innovative Trials
Regulatory bodies in India are increasingly supportive of innovative trial designs, which is positively impacting the clinical trials market. The introduction of adaptive trial designs and fast-track approvals for breakthrough therapies is encouraging pharmaceutical companies to conduct trials in India. In 2025, it is anticipated that the number of innovative trial designs will increase by 25%, reflecting the regulatory environment's responsiveness to industry needs. This support not only expedites the drug development process but also enhances the attractiveness of India as a destination for clinical trials. Consequently, the clinical trials market is likely to benefit from a more streamlined approval process, fostering a conducive environment for research and development.
Increasing Investment in Research and Development
The clinical trials market in India is experiencing a surge in investment in research and development (R&D). This trend is driven by both public and private sectors recognizing the potential of clinical trials to enhance drug development processes. In 2025, the Indian government allocated approximately $1.5 billion to support R&D initiatives, which is expected to bolster the clinical trials market. Furthermore, pharmaceutical companies are increasingly investing in innovative therapies, leading to a projected growth rate of 15% in the clinical trials market. This influx of capital is likely to facilitate the establishment of advanced clinical trial facilities and improve the overall infrastructure, thereby attracting more sponsors and enhancing the quality of trials conducted in India.
Rising Awareness and Participation in Clinical Trials
There is a notable increase in awareness and participation in clinical trials among the Indian population, which is a crucial driver for the clinical trials market. Educational initiatives and outreach programs are effectively informing patients about the benefits of participating in clinical trials, leading to a higher enrollment rate. In 2025, it is estimated that patient participation in clinical trials will rise by 15%, driven by improved understanding and trust in the clinical research process. This growing interest not only enhances the recruitment of diverse patient populations but also contributes to the overall success of clinical trials. As awareness continues to grow, the clinical trials market is expected to thrive, with more individuals willing to engage in research studies.