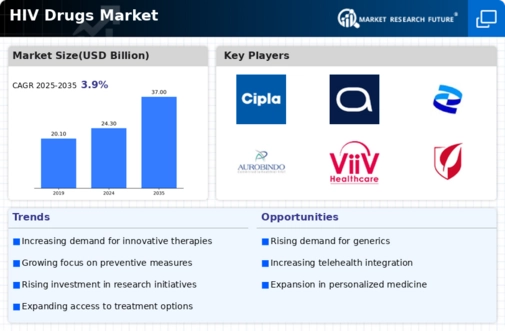
Top Industry Leaders in the HIV Drugs Market

Gilead Sciences (US) Received approvals for their long-acting injectable HIV treatment Sunlenca® (lenacapavir) in combination with other antiretrovirals, offering convenient and effective therapy options.Partnered with research institutions on developing a long-acting injectable combination of lenacapavir with islatravir for potential quarterly dosing.
Merck & Co. Completed Phase III trials for their investigational drug islatravir with potential advantages like once-daily dosing and fewer side effects, further diversifying their HIV treatment portfolio.Collaborated with advocacy groups to raise awareness about HIV testing and access to medications, particularly in marginalized communities.
ViiV Healthcare (UK) Introduced their Cabenuva® (Cabotegravir, Rilpivirine LA) long-acting injection for HIV treatment, providing a bimonthly dosing option for eligible patients.Focused on expanding global access to their HIV medications through partnerships with public health organizations and development agencies.
Janssen Pharmaceuticals (US) Received FDA approval for their new single-tablet regimen, Rukobia® (Fostemsavir, Tenofovir Alafenamide), simplifying treatment for individuals with multidrug-resistant HIV infections.Continued research on novel HIV prevention strategies, including long-acting injectable pre-exposure prophylaxis (PrEP) options.
Joint United Nations Programme on HIV/AIDS (UNAIDS) Launched the Fast-Track Cities initiative to accelerate progress towards ending AIDS in 30 high-burden cities by 2030, focusing on early diagnosis, treatment access, and social interventions.Advocated for increased global funding for HIV research and development, particularly for affordable and accessible treatment options in resource-limited settings.
List of Human Immunodeficiency Virus Drugs Key Companies in The Market
- Boehringer Ingelheim International GmbH (Germany)
- Cipla Inc. (India)
- Merck & Co., Inc. (US)
- AbbVie Inc. (US)
- Bristol-Myers Squibb Company (US)
- Teva Pharmaceutical Industries Ltd (Israel)
- Gilead Sciences, Inc. (US)
- Hoffmann-La Roche Ltd (Switzerland)
- Pfizer Inc. (US)
- Aurobindo Pharma (India)
- Celltrion Healthcare Co., Ltd (South Korea)
- ViiV Healthcare (UK)