Government Initiatives and Funding
Government initiatives aimed at combating hepatitis are significantly influencing the Hepatitis Test Solution Diagnosis Market. Various countries have launched national strategies to eliminate hepatitis as a public health threat, which includes increased funding for testing and treatment programs. For instance, initiatives that promote screening and vaccination are likely to enhance the accessibility of hepatitis testing solutions. The allocation of resources towards public health campaigns and the establishment of testing facilities are expected to create a favorable environment for market growth. As governments prioritize hepatitis elimination, the demand for innovative diagnostic solutions is anticipated to rise, thereby benefiting the industry.
Rising Demand for Home Testing Solutions
The trend towards home testing solutions is emerging as a significant driver in the Hepatitis Test Solution Diagnosis Market. With the growing preference for convenience and privacy, individuals are increasingly seeking at-home testing options for hepatitis. This shift is likely to be fueled by advancements in technology that allow for accurate and reliable testing outside of traditional healthcare settings. The availability of user-friendly home testing kits is expected to empower individuals to take charge of their health, leading to increased testing rates. As the market adapts to this demand, the development and distribution of home testing solutions are anticipated to expand, further contributing to the industry's growth.
Rising Prevalence of Hepatitis Infections
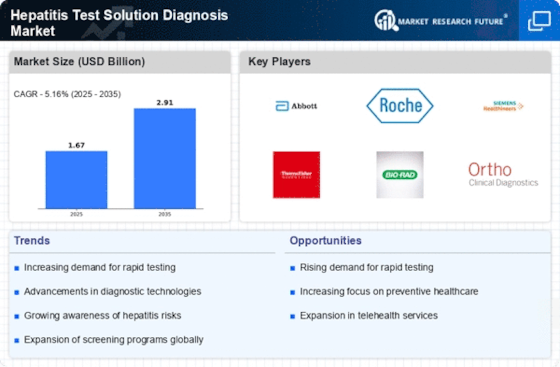
The increasing incidence of hepatitis infections worldwide is a primary driver for the Hepatitis Test Solution Diagnosis Market. According to health organizations, millions are affected by hepatitis B and C, necessitating effective diagnostic solutions. This rising prevalence is prompting healthcare systems to enhance their testing capabilities, thereby expanding the market. The World Health Organization estimates that approximately 325 million people are living with chronic hepatitis B and C infections, which underscores the urgent need for reliable testing solutions. As awareness grows regarding the importance of early detection and treatment, the demand for hepatitis testing solutions is likely to surge, further propelling the market forward.
Growing Awareness and Education on Hepatitis
The increasing awareness and education surrounding hepatitis are pivotal drivers for the Hepatitis Test Solution Diagnosis Market. Public health campaigns aimed at educating individuals about the risks and consequences of hepatitis are fostering a culture of proactive health management. As more people become informed about the importance of testing, the demand for hepatitis diagnostic solutions is likely to increase. Educational initiatives that highlight the benefits of early detection and treatment are essential in reducing stigma and encouraging individuals to seek testing. This heightened awareness is expected to translate into a greater uptake of hepatitis testing services, thereby propelling market growth.
Technological Innovations in Diagnostic Tools
Technological advancements in diagnostic tools are reshaping the Hepatitis Test Solution Diagnosis Market. Innovations such as point-of-care testing, rapid diagnostic tests, and molecular diagnostics are enhancing the accuracy and speed of hepatitis testing. These advancements not only improve patient outcomes but also facilitate early detection, which is crucial for effective management of the disease. The introduction of user-friendly and cost-effective testing solutions is likely to attract a broader demographic, including underserved populations. As technology continues to evolve, the market is expected to witness a surge in demand for sophisticated diagnostic tools that can provide timely and reliable results.