Advancements in Diagnostic Technologies
Technological innovations in diagnostic methodologies are significantly influencing the hepatitis test-solution-diagnosis market. The introduction of advanced molecular techniques, such as PCR and next-generation sequencing, has improved the accuracy and speed of hepatitis testing. These technologies enable the detection of viral RNA and DNA, facilitating early diagnosis and treatment initiation. Moreover, the integration of point-of-care testing devices is enhancing accessibility, particularly in rural areas of Japan where traditional laboratory facilities may be limited. As these technologies become more prevalent, they are likely to drive market growth by providing healthcare professionals with reliable tools for hepatitis diagnosis. The ongoing research and development efforts in this field suggest a promising future for the hepatitis test-solution-diagnosis market, as new innovations continue to emerge.
Growing Demand for Preventive Healthcare
The shift towards preventive healthcare is emerging as a significant driver for the hepatitis test-solution-diagnosis market. In Japan, there is an increasing recognition of the importance of early detection and prevention of diseases, including hepatitis. This trend is reflected in the rising number of health check-ups and screenings being conducted across the country. As individuals become more proactive about their health, the demand for hepatitis testing solutions is likely to increase. Furthermore, healthcare providers are focusing on preventive measures to reduce the burden of chronic diseases, which aligns with the objectives of the hepatitis test-solution-diagnosis market. This growing emphasis on prevention may lead to innovative testing solutions that cater to the needs of the population.
Rising Incidence of Hepatitis Infections
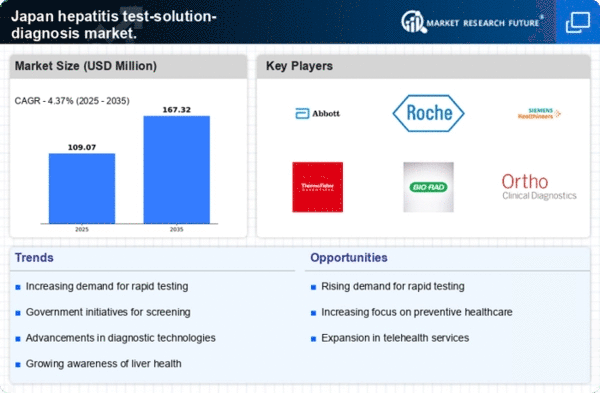
The increasing incidence of hepatitis infections in Japan is a critical driver for the hepatitis test-solution-diagnosis market. Recent data indicates that approximately 1.5 million individuals are living with chronic hepatitis B and C in Japan. This rising prevalence necessitates enhanced diagnostic solutions to identify and manage these infections effectively. As awareness grows regarding the health implications of hepatitis, healthcare providers are likely to invest more in testing solutions. The demand for accurate and rapid diagnostic tests is expected to surge, thereby propelling market growth. Furthermore, the aging population in Japan, which is more susceptible to viral hepatitis, adds to the urgency for effective testing solutions. Consequently, the hepatitis test-solution-diagnosis market is poised for expansion as healthcare systems adapt to these rising challenges.
Government Policies and Health Initiatives
Government policies and health initiatives play a pivotal role in shaping the hepatitis test-solution-diagnosis market. The Japanese government has implemented various programs aimed at increasing hepatitis awareness and promoting testing among at-risk populations. For instance, initiatives such as free screening programs and public health campaigns are designed to encourage individuals to get tested. These efforts are crucial in reducing the stigma associated with hepatitis and improving early detection rates. Additionally, funding allocated for hepatitis research and treatment further supports the market's growth. As the government continues to prioritize hepatitis management, the demand for effective diagnostic solutions is expected to rise, thereby benefiting the hepatitis test-solution-diagnosis market.
Increased Investment in Healthcare Infrastructure
Investment in healthcare infrastructure is a crucial factor driving the hepatitis test-solution-diagnosis market. Japan's healthcare system is undergoing significant enhancements, with a focus on improving diagnostic capabilities. The government and private sector are channeling funds into upgrading laboratory facilities and expanding access to testing services. This investment is likely to facilitate the adoption of advanced diagnostic technologies and improve the overall efficiency of hepatitis testing. Additionally, as healthcare facilities become more equipped to handle a higher volume of tests, the market for hepatitis test solutions is expected to grow. The ongoing commitment to strengthening healthcare infrastructure indicates a positive outlook for the hepatitis test-solution-diagnosis market in the coming years.