Rapid Diagnostics Market Summary
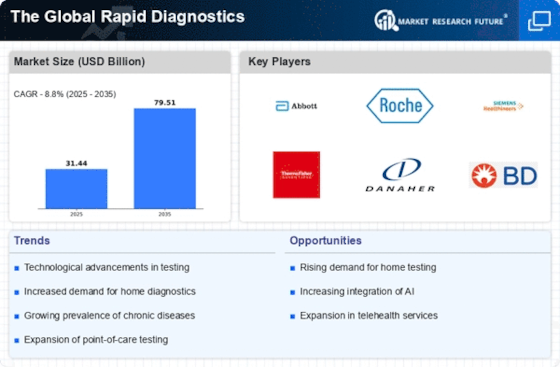
As per Market Research Future analysis, The Global Rapid Diagnostics Market was estimated at 31.44 USD Billion in 2024. The rapid diagnostics industry is projected to grow from 34.21 USD Billion in 2025 to 79.51 USD Billion by 2035, exhibiting a compound annual growth rate (CAGR) of 8% during the forecast period 2025 - 2035
Key Market Trends & Highlights
The Global Rapid Diagnostics Market is poised for substantial growth driven by technological advancements and a shift towards point-of-care testing.
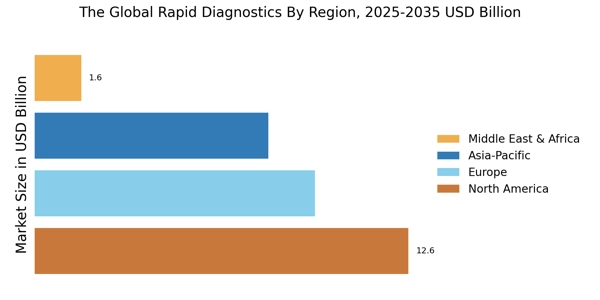
- North America remains the largest market for rapid diagnostics, reflecting a robust demand for innovative testing solutions.
- The Asia-Pacific region is emerging as the fastest-growing market, fueled by increasing healthcare investments and rising disease prevalence.
- Over-the-Counter (OTC) kits dominate the market, while professional kits are experiencing the fastest growth due to their enhanced accuracy and reliability.
- Technological advancements and a rising incidence of infectious diseases are key drivers propelling the market forward.
Market Size & Forecast
| 2024 Market Size | 31.44 (USD Billion) |
| 2035 Market Size | 79.51 (USD Billion) |
| CAGR (2025 - 2035) | 8.8% |
Major Players
Abbott Laboratories (US), Roche Diagnostics (CH), Siemens Healthineers (DE), Thermo Fisher Scientific (US), Danaher Corporation (US), BD (Becton, Dickinson and Company) (US), bioMérieux (FR), Cepheid (US), Hologic (US), Ortho Clinical Diagnostics (US)