Patient-Centric Approaches
The shift towards patient-centric approaches in healthcare is influencing the pharmacovigilance market in the GCC. There is a growing recognition of the importance of patient feedback in identifying and managing drug safety issues. This trend is prompting pharmaceutical companies to engage more actively with patients and healthcare providers to gather real-world data on drug effects. As a result, the demand for pharmacovigilance services that incorporate patient-reported outcomes is likely to rise. Market analysts suggest that this could lead to a 20% increase in the adoption of patient-centric pharmacovigilance solutions over the next few years, as companies strive to enhance their safety monitoring practices and improve patient trust.
Rising Demand for Drug Safety
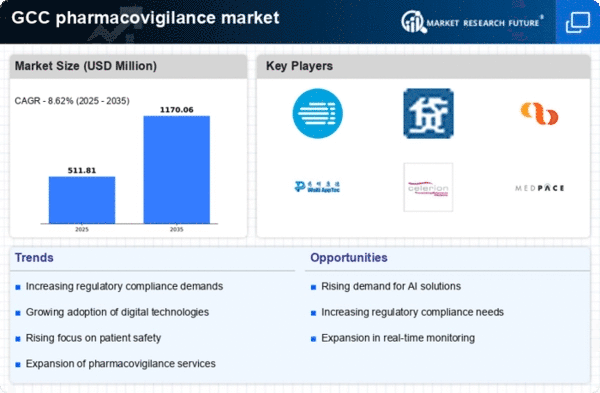
The increasing focus on drug safety within the pharmacovigilance market is driven by heightened regulatory scrutiny and public awareness. In the GCC region, regulatory bodies are emphasizing the importance of monitoring adverse drug reactions (ADRs) to ensure patient safety. This has led to a surge in demand for pharmacovigilance services, as pharmaceutical companies seek to comply with stringent regulations. The market is projected to grow at a CAGR of approximately 10% over the next five years, reflecting the urgent need for effective safety monitoring systems. As a result, organizations are investing in advanced pharmacovigilance solutions to enhance their safety profiles and mitigate risks associated with drug therapies.
Growing Pharmaceutical Industry
The expansion of the pharmaceutical industry in the GCC region is a significant driver for the pharmacovigilance market. As more drugs are developed and introduced, the need for robust pharmacovigilance systems becomes increasingly critical. The GCC pharmaceutical market is projected to reach $30 billion by 2026, which will likely necessitate enhanced safety monitoring practices. This growth is prompting pharmaceutical companies to prioritize pharmacovigilance to manage the risks associated with new drug launches. Furthermore, the increasing number of clinical trials in the region is expected to further fuel the demand for comprehensive pharmacovigilance services, ensuring that safety data is meticulously collected and analyzed.
Regulatory Framework Enhancements
Recent enhancements in the regulatory framework governing drug safety in the GCC are significantly impacting the pharmacovigilance market. Regulatory authorities are implementing more stringent guidelines for adverse event reporting and risk management, which compel pharmaceutical companies to adopt more rigorous pharmacovigilance practices. This shift is likely to drive market growth, as companies invest in compliance solutions to meet these new requirements. The introduction of electronic reporting systems and standardized data collection methods is expected to streamline processes, thereby improving the overall efficiency of pharmacovigilance operations. As a result, the market is anticipated to experience a steady increase in demand for regulatory compliance services.
Integration of Artificial Intelligence
The integration of artificial intelligence (AI) technologies into the pharmacovigilance market is transforming how data is analyzed and interpreted. AI-driven tools can process vast amounts of data, identifying patterns and potential safety signals more efficiently than traditional methods. In the GCC, the adoption of AI is expected to increase, with market analysts estimating a growth rate of around 15% in AI-related pharmacovigilance solutions. This technological advancement not only streamlines the reporting process but also enhances the accuracy of safety assessments. Consequently, pharmaceutical companies are likely to invest heavily in AI technologies to improve their pharmacovigilance capabilities and ensure compliance with evolving regulatory requirements.