Regulatory Framework Enhancements
The regulatory landscape for pharmaceuticals in France is evolving, with enhancements aimed at expediting the approval process for innovative therapies. The pegylated drugs market is likely to benefit from these regulatory improvements, which are designed to encourage the development and commercialization of new treatments. In 2025, it is anticipated that the average time for drug approval will decrease by 20%, facilitating quicker access to pegylated drugs for patients. This shift in the regulatory framework is expected to stimulate growth within the pegylated drugs market, as companies can bring their products to market more efficiently, ultimately benefiting patient care.
Rising Demand for Biopharmaceuticals
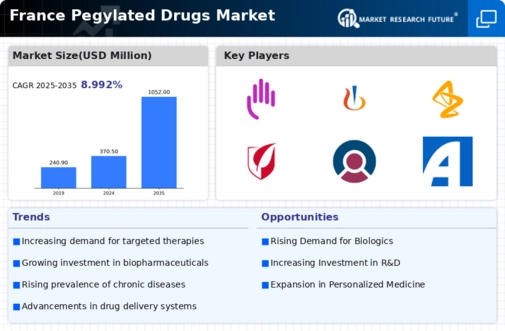
The pegylated drugs market in France is experiencing a notable surge in demand for biopharmaceuticals, driven by the increasing prevalence of chronic diseases such as cancer and autoimmune disorders. This trend is reflected in the growing number of pegylated formulations being developed, which enhance the therapeutic efficacy and safety profiles of existing drugs. In 2025, the biopharmaceutical sector is projected to account for approximately 30% of the total pharmaceutical market in France, indicating a robust growth trajectory. The pegylated drugs market is poised to benefit from this shift, as these innovative therapies offer improved patient outcomes and reduced side effects, making them more appealing to healthcare providers and patients alike.
Advancements in Drug Delivery Systems
Innovations in drug delivery systems are significantly impacting the pegylated drugs market in France. The development of advanced delivery mechanisms, such as nanoparticles and liposomes, enhances the bioavailability and stability of pegylated drugs. These advancements are crucial for improving the therapeutic effectiveness of treatments, particularly in oncology and chronic disease management. The pegylated drugs market is likely to see an increase in investment in research and development, with an estimated €500 million allocated to drug delivery innovations in 2025. This investment is expected to facilitate the introduction of new pegylated formulations that can better meet the needs of patients and healthcare providers.
Growing Focus on Personalized Medicine
The shift towards personalized medicine is becoming increasingly prominent within the pegylated drugs market in France. This approach tailors treatments to individual patient profiles, enhancing the efficacy of therapies. As healthcare providers seek to optimize treatment outcomes, the demand for pegylated drugs that can be customized to specific patient needs is likely to rise. The pegylated drugs market is expected to witness a growth rate of approximately 15% annually as personalized medicine becomes more integrated into clinical practice. This trend not only improves patient satisfaction but also aligns with the broader goals of healthcare systems to provide more effective and targeted therapies.
Increased Investment in Healthcare Infrastructure
The French government is making substantial investments in healthcare infrastructure, which is positively influencing the pegylated drugs market. Enhanced healthcare facilities and improved access to advanced therapies are expected to drive the adoption of pegylated drugs. In 2025, the French healthcare budget is projected to increase by 10%, with a significant portion allocated to innovative treatments. This investment is likely to facilitate better access to pegylated therapies, thereby expanding the market reach. The pegylated drugs market stands to gain from these developments, as improved infrastructure supports the distribution and administration of these advanced therapies.
















Leave a Comment