Aging Population
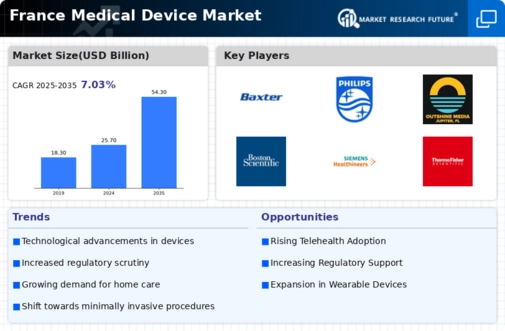
The aging population in France is a pivotal driver for the France medical device market. As the demographic shifts towards an older age group, the demand for medical devices that cater to chronic diseases and age-related health issues is likely to increase. According to recent statistics, approximately 20% of the French population is over 65 years old, a figure projected to rise significantly in the coming years. This demographic trend necessitates advanced medical devices, such as mobility aids, diagnostic equipment, and home healthcare solutions. Consequently, manufacturers are focusing on developing innovative products tailored to the needs of older adults, thereby expanding their market presence in the France medical device market.
Regulatory Framework
The regulatory framework governing the France medical device market plays a crucial role in shaping market dynamics. The European Union's Medical Device Regulation (MDR) has introduced stringent requirements for product approval and market entry, ensuring that devices meet high safety and efficacy standards. This regulatory evolution has prompted manufacturers to invest in compliance and quality assurance processes, which may initially increase costs but ultimately enhances consumer trust. Furthermore, the French National Agency for the Safety of Medicines and Health Products (ANSM) actively monitors the market, ensuring that only safe and effective devices are available to the public. This regulatory environment fosters a competitive landscape, encouraging innovation while safeguarding public health.
Technological Advancements
Technological advancements are transforming the landscape of the France medical device market. Innovations in areas such as telemedicine, wearable devices, and minimally invasive surgical technologies are reshaping patient care and treatment methodologies. For instance, the integration of artificial intelligence and machine learning in diagnostic tools enhances accuracy and efficiency, which is increasingly sought after by healthcare providers. The French government has been supportive of these advancements, investing in research and development initiatives that foster innovation. As a result, the market is witnessing a surge in the introduction of cutting-edge medical devices that improve patient outcomes and streamline healthcare processes.
Rising Healthcare Expenditure
Rising healthcare expenditure in France is a significant driver for the France medical device market. The French government allocates a substantial portion of its budget to healthcare, with spending projected to reach approximately 12% of GDP by 2026. This increase in funding facilitates the adoption of advanced medical technologies and devices across healthcare facilities. Hospitals and clinics are more likely to invest in state-of-the-art equipment, which in turn stimulates demand for innovative medical devices. Additionally, the emphasis on improving healthcare quality and accessibility further propels the growth of the medical device sector, as stakeholders seek to enhance patient care through the integration of new technologies.
Focus on Preventive Healthcare
The growing focus on preventive healthcare is emerging as a key driver in the France medical device market. With an increasing awareness of the importance of early diagnosis and disease prevention, there is a rising demand for diagnostic and monitoring devices. The French government has initiated various public health campaigns aimed at promoting preventive measures, which has led to a surge in the use of home monitoring devices and health apps. This trend is likely to encourage manufacturers to develop user-friendly and accessible medical devices that cater to the preventive healthcare segment. As a result, the France medical device market is expected to witness substantial growth in this area, aligning with the broader healthcare objectives of improving population health.