Advancements in Treatment Modalities
Innovations in treatment modalities for Focal Segmental Glomerulosclerosis are significantly influencing the Focal Segmental Glomerulosclerosis Treatment Market. Recent advancements in pharmacological therapies, such as the introduction of novel immunosuppressive agents and targeted therapies, have shown promise in improving patient outcomes. For instance, the development of agents that specifically target the underlying mechanisms of FSGS may enhance treatment efficacy and reduce side effects.
Additionally, the integration of personalized medicine approaches, which tailor treatments based on individual patient profiles, is gaining traction. This shift towards more effective and individualized treatment options is expected to drive market growth, as healthcare providers increasingly adopt these innovative therapies to manage FSGS more effectively.
Growing Awareness and Education Initiatives
The rising awareness and education initiatives surrounding Focal Segmental Glomerulosclerosis are playing a pivotal role in shaping the Focal Segmental Glomerulosclerosis Treatment Market. Increased efforts by healthcare organizations and advocacy groups to educate both healthcare professionals and patients about FSGS are leading to improved diagnosis and treatment rates.
These initiatives often include workshops, seminars, and informational campaigns that highlight the importance of early detection and management of kidney diseases. As awareness grows, more patients are likely to seek medical attention, resulting in a higher demand for treatment options. This trend is expected to contribute positively to the Focal Segmental Glomerulosclerosis Treatment Market, as healthcare providers respond to the increasing need for effective therapies.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies targeting Focal Segmental Glomerulosclerosis is emerging as a significant driver for the Focal Segmental Glomerulosclerosis Treatment Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for novel treatments that address unmet medical needs in FSGS.
This supportive regulatory environment encourages pharmaceutical companies to invest in the development of new therapies, as they can navigate the approval process more efficiently. Additionally, initiatives aimed at providing incentives for research and development in rare diseases, including FSGS, are likely to foster innovation. As a result, the Focal Segmental Glomerulosclerosis Treatment Market may witness a surge in the introduction of new and effective treatment options, ultimately benefiting patients.
Increased Research and Development Activities
The surge in research and development activities focused on Focal Segmental Glomerulosclerosis is a crucial driver for the Focal Segmental Glomerulosclerosis Treatment Market. Pharmaceutical companies and research institutions are increasingly investing in clinical trials aimed at discovering new therapeutic options and improving existing treatments. According to recent data, the number of clinical trials targeting FSGS has seen a notable increase, reflecting a growing commitment to addressing this complex condition.
This influx of research not only enhances the understanding of FSGS but also paves the way for the introduction of innovative therapies. As new treatments emerge from these research efforts, the Focal Segmental Glomerulosclerosis Treatment Market is likely to experience substantial growth, driven by the availability of more effective options for patients.
Rising Incidence of Focal Segmental Glomerulosclerosis
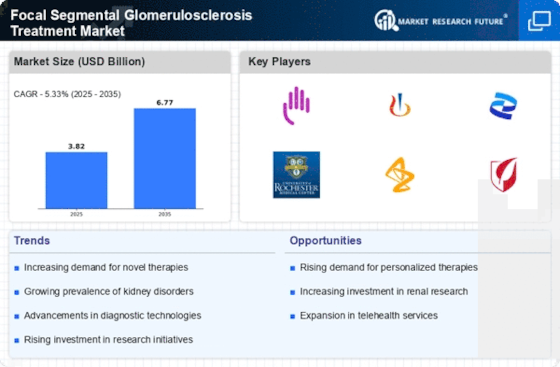
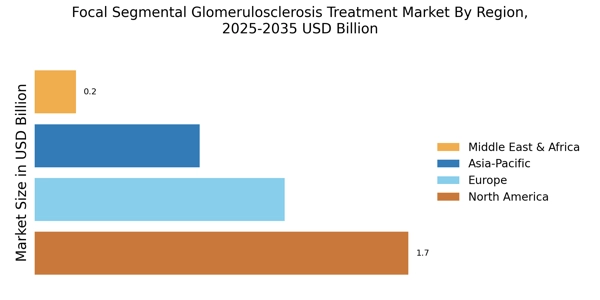
The increasing prevalence of Focal Segmental Glomerulosclerosis (FSGS) is a primary driver for the Focal Segmental Glomerulosclerosis Treatment Market. Recent studies indicate that the incidence of FSGS has been on the rise, with estimates suggesting that it affects approximately 7 to 10 individuals per million annually. This growing patient population necessitates the development and availability of effective treatment options, thereby propelling market growth.
As healthcare providers seek to address this rising burden, the demand for innovative therapies, including corticosteroids and immunosuppressants, is likely to increase. Furthermore, the heightened awareness of kidney diseases among healthcare professionals and patients may contribute to earlier diagnosis and treatment, further stimulating the Focal Segmental Glomerulosclerosis Treatment Market.