Increased Patient Awareness and Advocacy
The rise in patient awareness and advocacy is significantly impacting the Focal Segmental Glomerulosclerosis Drug Market. As patients become more informed about FSGS and its implications, there is a growing demand for effective treatments. Advocacy groups are playing a pivotal role in raising awareness, educating patients, and lobbying for better access to therapies. This heightened awareness is likely to lead to increased patient engagement in treatment decisions, which can influence market dynamics. Furthermore, as patients advocate for more research and funding, pharmaceutical companies may feel compelled to prioritize the development of new drugs for FSGS. This trend not only enhances the visibility of the disease but also encourages collaboration among stakeholders, ultimately contributing to the growth of the market.
Growing Investment in Renal Disease Research
The Focal Segmental Glomerulosclerosis Drug Market is experiencing growth due to increased investment in renal disease research. Governments and private organizations are recognizing the urgent need for effective treatments for kidney diseases, including FSGS. Funding for research initiatives has seen a notable rise, with millions allocated to studies aimed at understanding the underlying mechanisms of FSGS and developing new therapeutic strategies. This influx of capital is likely to accelerate the pace of innovation, leading to the discovery of novel drugs and treatment modalities. Furthermore, collaborations between academic institutions and pharmaceutical companies are becoming more common, enhancing the research landscape. As a result, the market is poised for expansion, with a greater number of effective therapies expected to emerge in the coming years.
Advancements in Drug Development Technologies
Technological advancements in drug development are significantly influencing the Focal Segmental Glomerulosclerosis Drug Market. Innovations such as high-throughput screening, artificial intelligence, and personalized medicine are streamlining the drug discovery process. These technologies enable researchers to identify potential drug candidates more efficiently, reducing the time and cost associated with bringing new therapies to market. For instance, the integration of biomarker-driven approaches allows for more precise targeting of FSGS, enhancing the likelihood of successful treatment outcomes. As a result, pharmaceutical companies are increasingly investing in these advanced technologies, which could lead to a surge in novel drug approvals. This trend not only fosters competition but also expands the therapeutic options available for patients suffering from FSGS.
Regulatory Incentives for Rare Disease Treatments
Regulatory incentives play a crucial role in shaping the Focal Segmental Glomerulosclerosis Drug Market. Authorities are increasingly providing support for the development of treatments for rare diseases, including FSGS. Programs such as orphan drug designations and fast-track approvals are designed to encourage pharmaceutical companies to invest in the development of therapies for conditions that affect a limited patient population. These incentives not only reduce the regulatory burden but also offer financial benefits, making it more attractive for companies to pursue research in this area. As a result, the number of drugs entering the market is likely to increase, providing patients with more options for managing their condition. This supportive regulatory environment is expected to foster innovation and drive growth within the FSGS drug market.
Rising Incidence of Focal Segmental Glomerulosclerosis
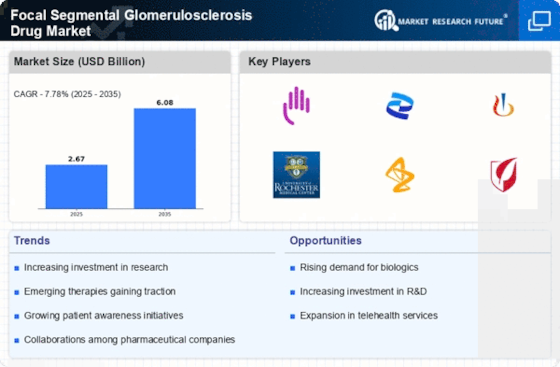
The increasing prevalence of Focal Segmental Glomerulosclerosis (FSGS) is a primary driver for the Focal Segmental Glomerulosclerosis Drug Market. Recent estimates suggest that FSGS affects approximately 7 to 10% of patients with end-stage renal disease. This rising incidence necessitates the development of effective therapeutic options, thereby propelling market growth. As awareness of kidney diseases expands, more patients are being diagnosed, which in turn increases the demand for specialized treatments. The growing patient population is likely to stimulate research and development efforts, leading to the introduction of innovative drugs. Consequently, pharmaceutical companies are focusing on creating targeted therapies that address the specific needs of FSGS patients, further enhancing the market landscape.