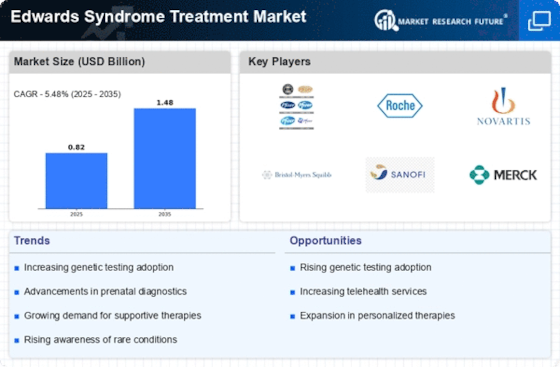
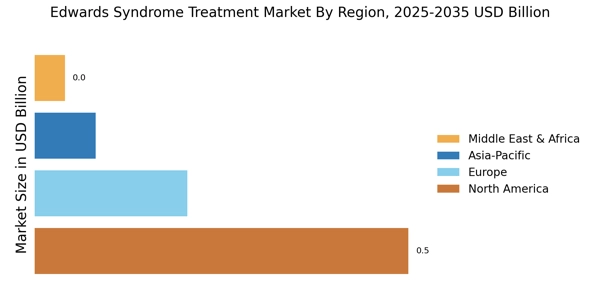
Advancements in Medical Technology
Technological advancements in medical devices and treatment methodologies are significantly influencing the Edwards Syndrome Treatment Market. Innovations such as non-invasive prenatal testing (NIPT) and advanced imaging techniques have improved the accuracy of diagnosis and monitoring of Edwards Syndrome. The market for prenatal testing has expanded, with estimates indicating a growth rate of around 15% annually. These advancements not only facilitate early detection but also enable healthcare providers to offer tailored treatment plans that address the specific needs of affected individuals. Additionally, the development of new therapeutic agents and surgical techniques is enhancing the quality of care for patients with Edwards Syndrome. As these technologies continue to evolve, they are expected to play a crucial role in shaping the future landscape of the Edwards Syndrome Treatment Market.
Rising Awareness of Genetic Disorders
The increasing awareness of genetic disorders, including Edwards Syndrome, is a pivotal driver for the Edwards Syndrome Treatment Market. Educational campaigns and advocacy groups are actively promoting understanding of genetic conditions, leading to earlier diagnosis and intervention. This heightened awareness is reflected in the growing number of genetic testing procedures, which have seen a rise of approximately 20% in recent years. As more families become informed about the implications of Edwards Syndrome, the demand for effective treatment options is likely to escalate, thereby propelling the market forward. Furthermore, healthcare providers are increasingly integrating genetic counseling into their practices, which may enhance patient engagement and treatment adherence. This trend suggests a promising outlook for the Edwards Syndrome Treatment Market as it aligns with the broader movement towards personalized medicine.
Regulatory Support for Treatment Approvals
Regulatory support for the approval of new treatments for rare diseases, including Edwards Syndrome, is a critical driver for the Edwards Syndrome Treatment Market. Regulatory agencies are increasingly recognizing the urgency of addressing unmet medical needs in rare conditions. Initiatives such as expedited review processes and orphan drug designations are facilitating faster access to innovative therapies. In recent years, the number of orphan drugs approved for rare diseases has increased significantly, suggesting a favorable environment for the development of treatments for Edwards Syndrome. This regulatory landscape not only encourages pharmaceutical companies to invest in research and development but also enhances the availability of effective treatment options for patients. As regulatory frameworks continue to evolve, they are likely to play a vital role in shaping the future of the Edwards Syndrome Treatment Market.
Growing Demand for Supportive Care Services
The increasing demand for supportive care services for families affected by Edwards Syndrome is a notable driver for the Edwards Syndrome Treatment Market. As awareness of the condition grows, families are seeking comprehensive care that encompasses not only medical treatment but also psychological and social support. This trend is evidenced by the rise in multidisciplinary care models, which integrate various healthcare professionals to address the diverse needs of patients and their families. The market for supportive care services is projected to grow at a rate of around 10% annually, driven by the need for holistic approaches to treatment. This shift towards comprehensive care is likely to enhance patient satisfaction and improve overall outcomes, thereby positively impacting the Edwards Syndrome Treatment Market.
Increased Funding for Rare Disease Research
The surge in funding for research on rare diseases, including Edwards Syndrome, is a significant driver for the Edwards Syndrome Treatment Market. Government and private sector investments have been directed towards understanding the genetic and molecular underpinnings of rare conditions. In recent years, funding for rare disease research has increased by approximately 25%, reflecting a growing recognition of the need for effective treatments. This influx of resources is likely to accelerate the development of novel therapeutic options and improve patient outcomes. Furthermore, collaborations between academic institutions and pharmaceutical companies are becoming more common, fostering innovation in treatment strategies. As research continues to advance, the Edwards Syndrome Treatment Market stands to benefit from the resulting breakthroughs and enhanced therapeutic offerings.