Advancements in Gene Therapy
Recent advancements in gene therapy are poised to revolutionize the Dystrophic Epidermolysis Bullosa Treatment Market. Innovative approaches, such as CRISPR and other gene-editing technologies, are being explored to correct the underlying genetic defects responsible for DEB. These therapies hold the promise of not only treating symptoms but also addressing the root cause of the disease. Clinical trials are currently underway, with some showing promising results in improving skin integrity and reducing blister formation. The potential for gene therapy to provide long-lasting solutions could significantly alter the treatment landscape for DEB. As these therapies progress through regulatory pathways, they are likely to attract substantial investment and interest from both pharmaceutical companies and investors, further driving market growth.
Growing Awareness and Advocacy
The growing awareness and advocacy surrounding Dystrophic Epidermolysis Bullosa are significantly influencing the Dystrophic Epidermolysis Bullosa Treatment Market. Advocacy groups and patient organizations are actively working to educate the public and healthcare providers about DEB, its challenges, and the need for effective treatments. This heightened awareness is leading to increased funding for research and development, as well as greater participation in clinical trials. Furthermore, as more individuals become informed about DEB, there is a corresponding rise in demand for specialized care and treatment options. This trend is likely to encourage pharmaceutical companies to prioritize DEB in their research agendas, ultimately resulting in a broader array of therapeutic solutions for patients.
Increased Investment in Rare Disease Research
The Dystrophic Epidermolysis Bullosa Treatment Market is benefiting from a surge in investment directed towards rare disease research. Governments and private organizations are recognizing the need for effective treatments for rare conditions like DEB, leading to increased funding for research and development. In recent years, funding for rare disease initiatives has seen a notable rise, with billions allocated to support innovative therapies. This influx of capital is facilitating the exploration of novel treatment modalities, including biologics and advanced wound care products. As a result, the market is witnessing a diversification of treatment options, which may enhance patient outcomes and satisfaction. The growing focus on rare diseases is likely to sustain momentum in the DEB treatment market, fostering an environment conducive to innovation.
Technological Innovations in Treatment Delivery
Technological innovations in treatment delivery are emerging as a crucial driver for the Dystrophic Epidermolysis Bullosa Treatment Market. Advances in medical technology, such as telemedicine and wearable devices, are enhancing patient access to care and improving treatment adherence. These innovations facilitate remote monitoring and management of DEB, allowing healthcare providers to offer timely interventions. Additionally, the development of advanced wound care technologies, including bioengineered skin substitutes, is transforming the way DEB is treated. These products not only promote healing but also reduce the frequency of painful dressing changes. As technology continues to evolve, it is likely to play an increasingly vital role in the management of DEB, thereby driving market growth and improving patient outcomes.
Rising Prevalence of Dystrophic Epidermolysis Bullosa
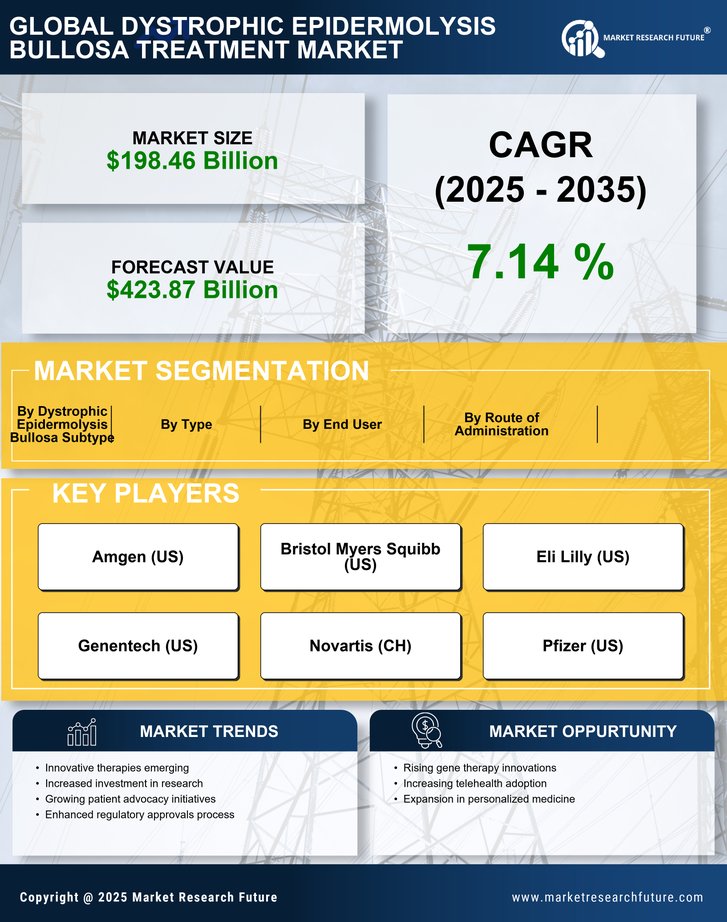
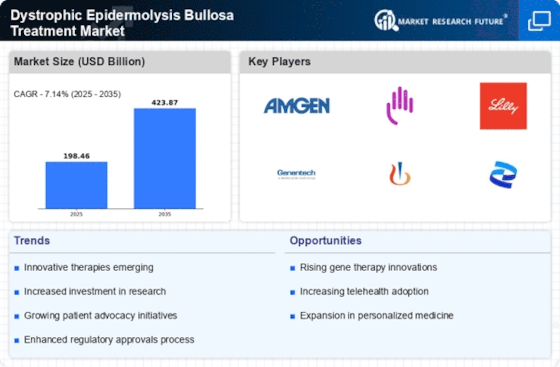
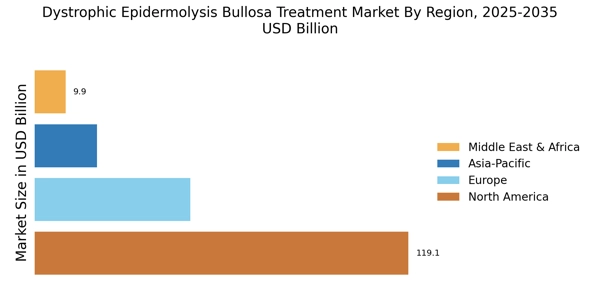
The increasing incidence of Dystrophic Epidermolysis Bullosa (DEB) is a primary driver for the Dystrophic Epidermolysis Bullosa Treatment Market. Recent estimates suggest that DEB affects approximately 1 in 50,000 live births, leading to a growing patient population requiring specialized care. This rising prevalence necessitates the development of innovative treatment options, thereby stimulating market growth. As awareness of DEB increases among healthcare professionals and the general public, more patients are likely to seek diagnosis and treatment. Consequently, pharmaceutical companies and research institutions are investing in the development of targeted therapies and advanced wound care solutions. This trend indicates a robust demand for effective treatments, which could potentially enhance the quality of life for patients suffering from this debilitating condition.