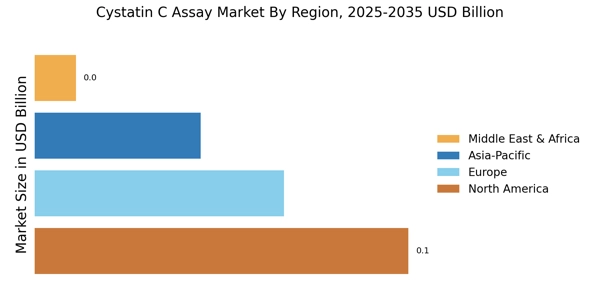
Growing Geriatric Population
The increasing geriatric population is a significant factor driving the Cystatin C Assay Market. As individuals age, the risk of developing kidney-related issues escalates, necessitating effective monitoring and diagnostic tools. The World Health Organization projects that the number of people aged 60 years and older will double by 2050, highlighting the urgent need for healthcare solutions tailored to this demographic. Cystatin C assays are particularly valuable in this context, as they provide a reliable means of assessing renal function in older adults, who may have atypical presentations of kidney disease. This demographic shift is likely to lead to heightened demand for Cystatin C testing, as healthcare systems adapt to the needs of an aging population. Consequently, the market is expected to expand, driven by the imperative to ensure optimal kidney health in older individuals.
Increasing Prevalence of Kidney Diseases
The rising incidence of kidney diseases is a primary driver for the Cystatin C Assay Market. Chronic kidney disease (CKD) has become a pressing health concern, with millions affected worldwide. According to recent data, the prevalence of CKD is estimated to be around 10-15% of the adult population, which underscores the urgent need for effective diagnostic tools. Cystatin C, as a biomarker, offers a more accurate assessment of kidney function compared to traditional methods. This growing recognition of Cystatin C's utility in early diagnosis and monitoring of kidney diseases is likely to propel the demand for assays in the market. As healthcare providers increasingly adopt Cystatin C testing, the market is expected to witness substantial growth, driven by the need for timely interventions and improved patient outcomes.
Regulatory Support for Biomarker Testing
Regulatory bodies are increasingly endorsing the use of biomarkers in clinical practice, which positively impacts the Cystatin C Assay Market. Guidelines from health authorities are evolving to incorporate biomarker testing as a standard practice for diagnosing and managing various conditions, including kidney diseases. This regulatory support not only legitimizes the use of Cystatin C assays but also encourages healthcare providers to adopt these tests in routine clinical settings. As more clinical studies validate the efficacy of Cystatin C as a biomarker for renal function, the market is likely to see a surge in demand. Furthermore, the establishment of clear regulatory pathways for assay approval may facilitate the entry of new players into the market, fostering competition and innovation. This supportive regulatory environment is expected to enhance the overall growth trajectory of the Cystatin C Assay Market.
Rising Awareness of Biomarkers in Diagnostics
There is a notable increase in awareness regarding the importance of biomarkers in disease diagnostics, which significantly influences the Cystatin C Assay Market. Healthcare professionals and patients alike are becoming more informed about the role of biomarkers in early disease detection and management. Cystatin C, recognized for its sensitivity in detecting renal impairment, is gaining traction as a preferred biomarker. This shift in perception is supported by various studies indicating that Cystatin C levels correlate more closely with kidney function than creatinine levels. Consequently, the demand for Cystatin C assays is expected to rise as healthcare systems prioritize precision medicine and personalized treatment approaches. The growing emphasis on biomarker-driven diagnostics is likely to enhance the market landscape, fostering innovation and expanding the range of available assays.
Technological Innovations in Assay Development
Technological advancements in assay development are significantly shaping the Cystatin C Assay Market. Innovations such as high-throughput screening, point-of-care testing, and automated systems are enhancing the efficiency and accuracy of Cystatin C assays. These advancements not only streamline the testing process but also reduce turnaround times, making it easier for healthcare providers to obtain timely results. Furthermore, the integration of advanced technologies, such as microfluidics and nanotechnology, is expected to improve assay sensitivity and specificity. As a result, the market is likely to experience increased adoption of Cystatin C assays, driven by the demand for rapid and reliable diagnostic solutions. The continuous evolution of assay technologies is anticipated to create new opportunities for market players, fostering competition and innovation.
.png)