Regulatory Approvals and Market Access
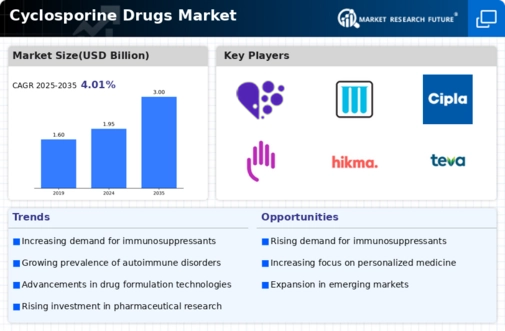
The landscape of regulatory approvals is a significant driver for the Cyclosporine Drugs Market. As regulatory bodies continue to streamline the approval process for new formulations and generics of cyclosporine, market access is likely to improve. This trend is particularly relevant as healthcare systems seek cost-effective treatment options for patients requiring immunosuppressive therapy. The introduction of generic versions of cyclosporine has the potential to increase market competition, leading to lower prices and improved accessibility for patients. As more products gain regulatory approval, the cyclosporine market is expected to experience growth, driven by enhanced availability and affordability of these essential medications.
Rising Incidence of Autoimmune Disorders
The growing prevalence of autoimmune disorders is significantly influencing the Cyclosporine Drugs Market. Conditions such as rheumatoid arthritis, lupus, and psoriasis are becoming increasingly common, leading to a heightened demand for effective treatment options. Cyclosporine, known for its immunosuppressive properties, is often prescribed to manage these conditions. Recent statistics indicate that autoimmune diseases affect millions of individuals worldwide, creating a substantial market for therapies that can alleviate symptoms and improve quality of life. As awareness of these disorders increases and diagnostic capabilities improve, the market for cyclosporine is expected to expand, driven by the need for reliable treatment solutions.
Increasing Prevalence of Organ Transplants
The rising incidence of organ transplants is a pivotal driver for the Cyclosporine Drugs Market. As more individuals require organ transplants due to conditions such as kidney failure, liver disease, and heart issues, the demand for immunosuppressive therapies, including cyclosporine, escalates. According to recent data, the number of organ transplants performed annually has shown a steady increase, with thousands of procedures conducted each year. This trend necessitates effective immunosuppressive agents to prevent organ rejection, thereby propelling the growth of the cyclosporine market. Furthermore, advancements in transplant techniques and post-operative care are likely to enhance patient outcomes, further driving the need for cyclosporine and similar drugs in the market.
Technological Advancements in Drug Delivery Systems
Innovations in drug delivery systems are emerging as a crucial driver for the Cyclosporine Drugs Market. Enhanced formulations and delivery mechanisms, such as nanotechnology and sustained-release systems, are being developed to improve the efficacy and safety of cyclosporine. These advancements aim to optimize therapeutic outcomes while minimizing side effects, which is particularly important given the drug's immunosuppressive nature. The introduction of novel delivery methods could potentially increase patient adherence and satisfaction, thereby expanding the market. As pharmaceutical companies invest in research and development to create more effective formulations, the cyclosporine market is likely to benefit from these technological improvements.
Growing Awareness and Education on Immunosuppressive Therapies
The increasing awareness and education surrounding immunosuppressive therapies are playing a vital role in the Cyclosporine Drugs Market. Healthcare professionals and patients are becoming more informed about the benefits and risks associated with cyclosporine treatment. This heightened awareness is leading to more proactive management of conditions requiring immunosuppression, such as organ transplants and autoimmune diseases. Educational initiatives and patient support programs are helping to demystify the use of cyclosporine, encouraging its adoption among patients who may benefit from its use. As understanding of these therapies grows, the market for cyclosporine is expected to expand, driven by informed patient choices and physician recommendations.