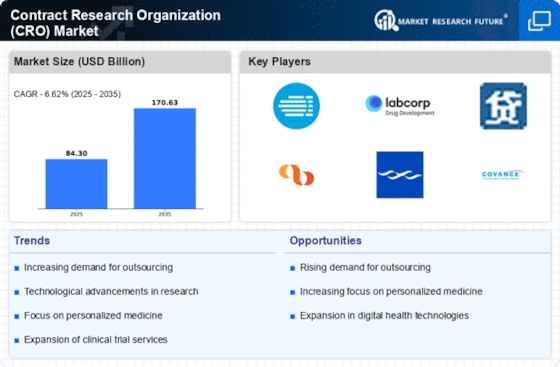
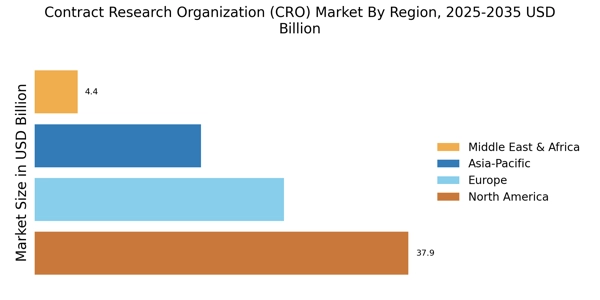
Rising Demand for Drug Development
The Contract Research Organization Market experiences a notable increase in demand for drug development services. This trend is driven by the growing need for innovative therapies and the rising prevalence of chronic diseases. According to recent data, the pharmaceutical sector is projected to invest over 200 billion dollars in research and development by 2026. Consequently, Contract Research Organizations are positioned to play a pivotal role in facilitating clinical trials and ensuring timely drug approvals. The increasing complexity of clinical trials necessitates specialized expertise, which further propels the demand for these organizations. As pharmaceutical companies seek to streamline their operations and reduce costs, the reliance on Contract Research Organizations for drug development is likely to intensify, thereby shaping the future landscape of the industry.
Expansion of Biopharmaceutical Sector
The biopharmaceutical sector's expansion significantly influences the Contract Research Organization Market. With advancements in biotechnology and personalized medicine, there is a surge in the development of biologics and biosimilars. This sector is expected to grow at a compound annual growth rate of approximately 8% through 2026. As biopharmaceutical companies increasingly outsource their research activities to Contract Research Organizations, the latter are becoming essential partners in navigating the complexities of biologic drug development. The need for specialized knowledge in areas such as cell and gene therapy further underscores the importance of these organizations. This trend not only enhances the efficiency of drug development processes but also fosters innovation within the biopharmaceutical landscape.
Increased Focus on Patient-Centric Trials
The Contract Research Organization Market is witnessing a shift towards patient-centric clinical trials. This approach emphasizes the importance of patient engagement and experience in the research process. As regulatory bodies advocate for more inclusive trial designs, Contract Research Organizations are adapting their methodologies to incorporate patient feedback and preferences. This trend is likely to enhance recruitment and retention rates, ultimately leading to more successful trial outcomes. Furthermore, the integration of digital health technologies facilitates real-time data collection and patient monitoring, which aligns with the patient-centric model. As the industry evolves, the ability of Contract Research Organizations to implement these strategies will be crucial in meeting the demands of both sponsors and regulatory authorities.
Technological Advancements in Data Management
Technological advancements are reshaping the Contract Research Organization Market, particularly in data management and analytics. The adoption of artificial intelligence and machine learning is streamlining data collection, analysis, and reporting processes. These technologies enable Contract Research Organizations to handle large volumes of data efficiently, thereby improving the accuracy and speed of clinical trial results. As the industry moves towards more data-driven decision-making, organizations that leverage these technologies are likely to gain a competitive edge. Moreover, the integration of cloud-based solutions enhances collaboration among stakeholders, facilitating real-time access to critical information. This technological evolution not only optimizes operational efficiency but also enhances the overall quality of research outcomes.
Regulatory Changes and Compliance Requirements
The Contract Research Organization Market is significantly impacted by evolving regulatory changes and compliance requirements. As governments and regulatory bodies implement stricter guidelines for clinical trials, the demand for expertise in regulatory affairs is increasing. Contract Research Organizations are tasked with ensuring that their clients adhere to these regulations, which can vary across regions. This complexity necessitates a robust understanding of local and international compliance standards. Organizations that can navigate these regulatory landscapes effectively are likely to thrive in the competitive market. Additionally, the emphasis on quality assurance and risk management further underscores the importance of compliance in clinical research. As the industry adapts to these changes, the role of Contract Research Organizations in facilitating compliance will become increasingly vital.