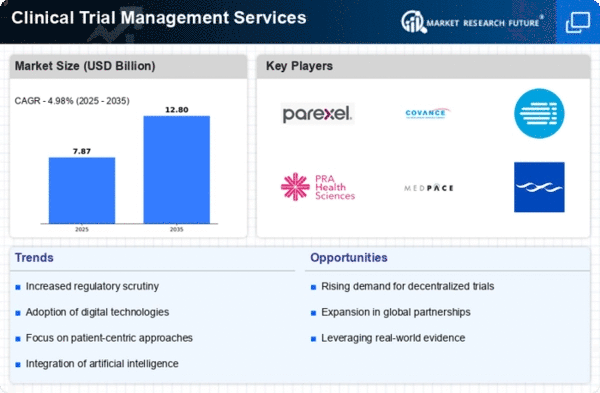
The Clinical Trial Management Services market is characterized by a dynamic competitive landscape, driven by the increasing demand for efficient and effective clinical trials. Key players are focusing on innovation, digital transformation, and strategic partnerships to enhance their service offerings. Companies such as Parexel International (US), Covance (US), and IQVIA (US) are at the forefront, leveraging advanced technologies and data analytics to streamline trial processes and improve patient engagement. Their collective strategies not only enhance operational efficiency but also shape the competitive environment by setting higher standards for service delivery and patient-centric approaches.In terms of business tactics, companies are increasingly localizing their operations to better cater to regional needs, optimizing supply chains to reduce costs, and enhancing their technological capabilities. The market appears moderately fragmented, with a mix of large multinational corporations and smaller specialized firms. This structure allows for a diverse range of services, although the influence of major players like PRA Health Sciences (US) and Medpace (US) is significant, as they continue to expand their global footprint and service capabilities.
In November Parexel International (US) announced a strategic partnership with a leading AI technology firm to enhance its data analytics capabilities. This collaboration aims to integrate AI-driven insights into clinical trial design and execution, potentially reducing trial timelines and improving patient recruitment. Such a move underscores Parexel's commitment to innovation and positions it favorably in a competitive market increasingly reliant on technology.
In October Covance (US) launched a new digital platform designed to facilitate remote patient monitoring during clinical trials. This initiative reflects a growing trend towards decentralized trials, which can enhance patient participation and data collection efficiency. By adopting this technology, Covance not only addresses current market demands but also strengthens its competitive edge by offering more flexible trial solutions.
In September IQVIA (US) expanded its global reach by acquiring a regional clinical research organization in Asia. This acquisition is likely to enhance IQVIA's capabilities in conducting trials in emerging markets, where there is a rising demand for clinical research services. The strategic importance of this move lies in IQVIA's ability to leverage local expertise and resources, thereby improving its service delivery in diverse geographical regions.
As of December the Clinical Trial Management Services market is witnessing trends such as digitalization, sustainability, and the integration of AI technologies. Strategic alliances are increasingly shaping the competitive landscape, enabling companies to pool resources and expertise. The shift from price-based competition to a focus on innovation, technology, and supply chain reliability is evident. Moving forward, competitive differentiation will likely hinge on the ability to adapt to these trends, with companies that prioritize technological advancements and patient-centric solutions poised to lead the market.