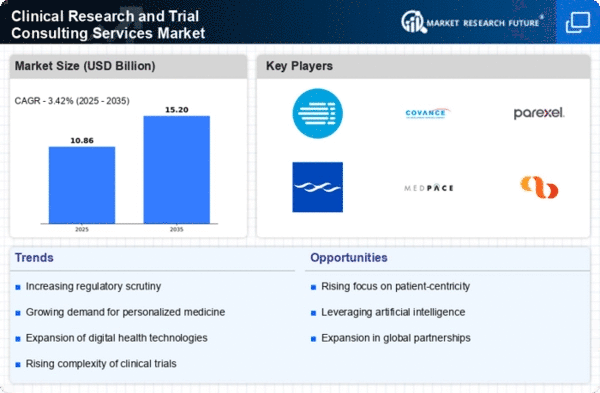
The Clinical Research and Trial Consulting Services Market is currently experiencing a dynamic evolution, driven by the increasing complexity of clinical trials and the growing demand for innovative therapies. As pharmaceutical and biotechnology companies seek to navigate regulatory landscapes and optimize trial designs, the role of consulting services becomes increasingly pivotal. These services not only assist in ensuring compliance with stringent regulations but also enhance the efficiency of trial execution. Furthermore, the integration of advanced technologies, such as artificial intelligence and data analytics, is reshaping the landscape, enabling more precise patient recruitment and improved trial outcomes. This transformation suggests a shift towards more personalized and adaptive trial methodologies, which could potentially lead to faster drug development cycles and reduced costs.
In addition, the market appears to be influenced by a rising emphasis on patient-centric approaches. Stakeholders are recognizing the importance of patient engagement and experience in clinical trials, which may lead to higher retention rates and more reliable data. As a result, consulting firms are likely to adapt their strategies to incorporate patient feedback and preferences into trial designs. This trend indicates a broader movement towards collaboration among various stakeholders, including sponsors, regulatory bodies, and patients, fostering a more holistic approach to clinical research. Overall, the Clinical Research and Trial Consulting Services Market is poised for continued growth, driven by innovation, collaboration, and a focus on patient outcomes.
Integration of Technology
The Clinical Research and Trial Consulting Services Market is witnessing a notable trend towards the integration of advanced technologies. This includes the use of artificial intelligence, machine learning, and data analytics to streamline trial processes. Such technologies facilitate better patient recruitment, enhance data management, and improve overall trial efficiency. As a result, consulting services are increasingly leveraging these tools to provide more effective solutions to their clients.
Patient-Centric Approaches
There is a growing emphasis on patient-centric approaches within the Clinical Research and Trial Consulting Services Market. Stakeholders are recognizing the value of incorporating patient feedback and preferences into trial designs. This trend aims to enhance patient engagement and retention, ultimately leading to more reliable data and improved trial outcomes. Consulting firms are adapting their strategies to prioritize the patient experience.
Regulatory Compliance and Adaptation
The Clinical Research and Trial Consulting Services Market is also characterized by a heightened focus on regulatory compliance. As regulations evolve, consulting services are essential in helping clients navigate these changes effectively. This trend underscores the importance of staying informed about regulatory requirements and adapting trial designs accordingly, ensuring that studies meet the necessary standards for approval.