Consumer Awareness and Advocacy
Consumer awareness and advocacy regarding drug safety are becoming increasingly influential in China, impacting the pharmaceutical quality-control market. As patients become more informed about their medications, they are demanding higher standards of quality and transparency from pharmaceutical companies. This shift in consumer behavior is prompting manufacturers to enhance their quality control processes to maintain market competitiveness. Surveys indicate that over 70% of consumers prioritize quality assurance when selecting pharmaceutical products. Consequently, companies are likely to invest more in quality control measures to align with consumer expectations, thereby driving growth in the pharmaceutical quality-control market.
Stringent Regulatory Frameworks
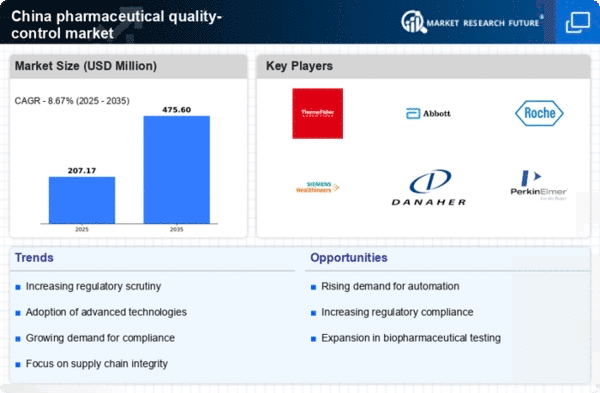
The stringent regulatory frameworks established by the Chinese government are a crucial driver for the pharmaceutical quality-control market. Regulatory bodies such as the National Medical Products Administration (NMPA) enforce rigorous standards for drug safety and efficacy. Compliance with these regulations is mandatory for pharmaceutical companies, which necessitates robust quality control measures. The market is responding to these requirements, with investments in quality management systems and testing facilities increasing. It is estimated that adherence to these regulations could account for up to 30% of operational costs for pharmaceutical manufacturers. As a result, the pharmaceutical quality-control market is likely to expand as companies strive to meet these evolving regulatory demands.
Growing Biopharmaceutical Sector
The rapid growth of the biopharmaceutical sector in China is driving the pharmaceutical quality-control market. With an increasing number of biopharmaceutical products entering the market, there is a heightened need for specialized quality control measures. Biopharmaceuticals often require more complex testing and validation processes compared to traditional pharmaceuticals, which can lead to increased demand for quality-control services. The biopharmaceutical market in China is projected to grow at a CAGR of approximately 15% over the next five years, further emphasizing the need for stringent quality control. This growth presents opportunities for companies specializing in quality assurance, thereby bolstering the pharmaceutical quality-control market.
Rising Demand for Quality Assurance
The increasing demand for high-quality pharmaceuticals in China is a primary driver for the pharmaceutical quality-control market. As the population becomes more health-conscious, there is a growing expectation for safe and effective medications. This trend is reflected in the market, which is projected to reach approximately $3 billion by 2026. Pharmaceutical companies are investing heavily in quality assurance processes to meet these expectations, ensuring that their products comply with stringent safety standards. The emphasis on quality assurance not only enhances consumer trust but also helps companies avoid costly recalls and legal issues. Consequently, the pharmaceutical quality-control market will experience robust growth as manufacturers prioritize quality in their production processes..
Technological Advancements in Testing
Technological advancements are significantly influencing the pharmaceutical quality-control market in China. Innovations such as automated testing systems, real-time monitoring, and advanced analytical techniques are enhancing the efficiency and accuracy of quality control processes. For instance, the integration of artificial intelligence and machine learning in quality testing is streamlining operations and reducing human error. This shift towards automation is expected to increase the market's value, with estimates suggesting a growth rate of around 10% annually over the next five years. As companies adopt these technologies, they are likely to improve their compliance with regulatory standards, thereby strengthening their position in the competitive landscape of the pharmaceutical quality-control market.