Market Growth Projections
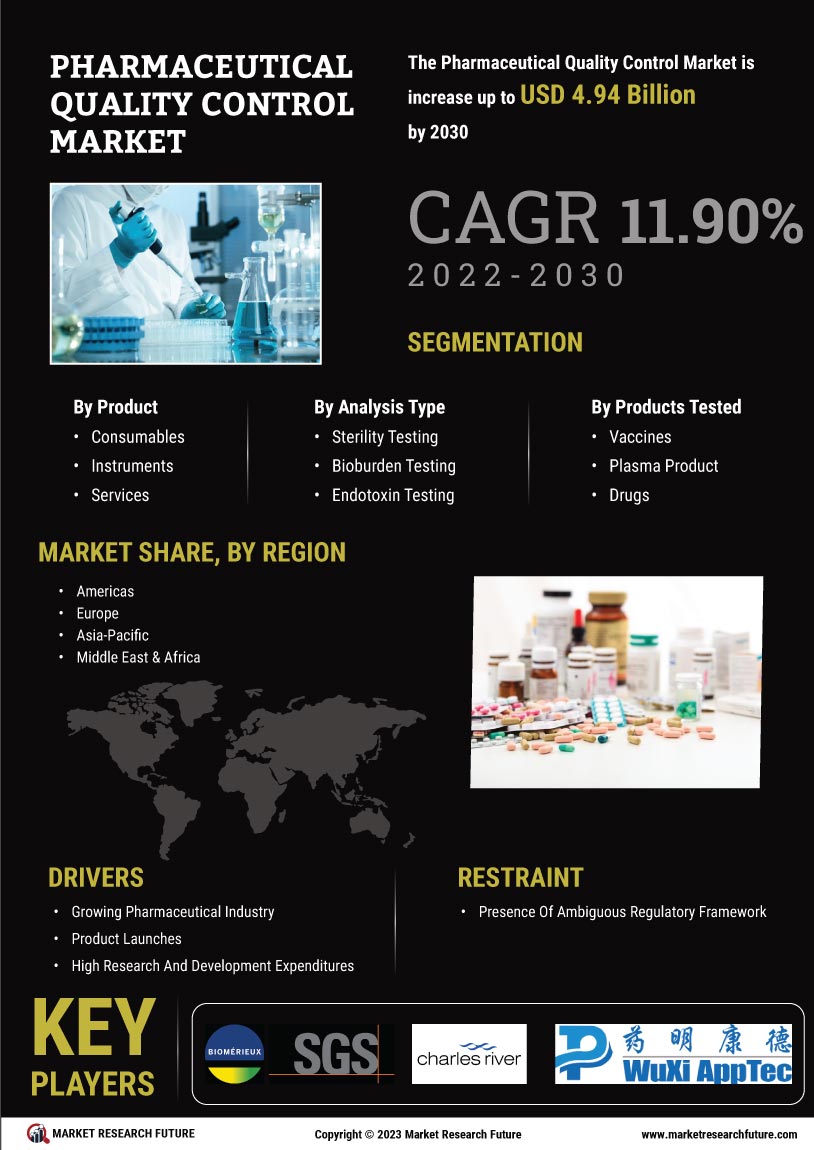
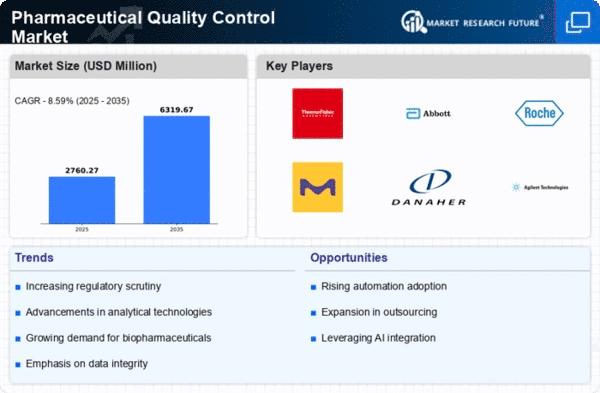
The Global Pharmaceutical Quality Control Market Industry is poised for substantial growth, with projections indicating a market value of 5.16 USD Billion in 2024 and an anticipated increase to 16.2 USD Billion by 2035. This growth trajectory suggests a compound annual growth rate (CAGR) of 10.96% from 2025 to 2035. The factors driving this growth include rising regulatory compliance, technological advancements, increasing demand for biopharmaceuticals, globalization of supply chains, and growing consumer awareness. These dynamics collectively indicate a robust future for the quality control sector within the pharmaceutical industry.
Rising Regulatory Compliance
The Global Pharmaceutical Quality Control Market Industry is experiencing heightened scrutiny from regulatory bodies, necessitating stringent adherence to quality standards. Regulatory agencies such as the FDA and EMA impose rigorous guidelines that pharmaceutical companies must follow to ensure product safety and efficacy. This compliance is crucial, as non-compliance can lead to severe penalties and product recalls. As a result, companies are investing significantly in quality control measures, which is projected to drive the market value to 5.16 USD Billion in 2024. The emphasis on regulatory compliance is likely to sustain growth in the industry, fostering innovations in quality assurance technologies.
Increasing Demand for Biopharmaceuticals
The Global Pharmaceutical Quality Control Market Industry is witnessing a surge in demand for biopharmaceuticals, which necessitates robust quality control measures. Biopharmaceuticals, including monoclonal antibodies and vaccines, require stringent quality assessments to ensure their safety and effectiveness. As the biopharmaceutical sector expands, driven by advancements in biotechnology, the need for specialized quality control processes becomes paramount. This trend is likely to contribute to the market's growth, as companies invest in sophisticated quality control systems to meet the unique challenges posed by biopharmaceuticals. The industry's evolution is expected to align with the projected CAGR of 10.96% from 2025 to 2035.
Globalization of Pharmaceutical Supply Chains
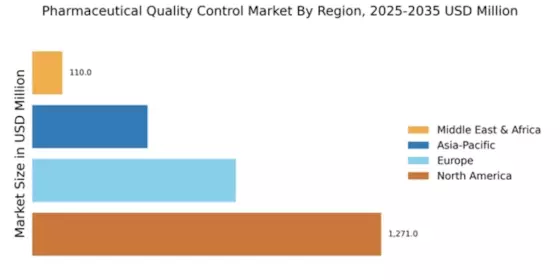
The globalization of pharmaceutical supply chains is significantly impacting the Global Pharmaceutical Quality Control Market Industry. As companies source raw materials and components from various regions, ensuring consistent quality across the supply chain becomes increasingly complex. This globalization necessitates the implementation of comprehensive quality control systems to monitor and manage quality at every stage of production. Companies are compelled to adopt standardized quality practices to mitigate risks associated with supply chain variability. This trend is likely to drive investments in quality control technologies and processes, further propelling market growth in the coming years.
Technological Advancements in Quality Control
Technological innovations are transforming the Global Pharmaceutical Quality Control Market Industry, enhancing the efficiency and accuracy of quality assessments. Advanced technologies such as artificial intelligence, machine learning, and automation are being integrated into quality control processes. These technologies facilitate real-time monitoring and data analysis, allowing for quicker decision-making and improved product quality. As companies adopt these innovations, the market is expected to grow significantly, with projections indicating a rise to 16.2 USD Billion by 2035. The ongoing technological evolution is likely to create a competitive edge for organizations that prioritize quality control in their operations.
Growing Consumer Awareness and Safety Concerns
Consumer awareness regarding pharmaceutical product safety is on the rise, influencing the Global Pharmaceutical Quality Control Market Industry. Patients and healthcare providers are increasingly demanding transparency and assurance of product quality. This heightened awareness drives pharmaceutical companies to prioritize quality control measures to maintain consumer trust and meet regulatory expectations. As a result, organizations are investing in quality assurance programs and certifications to demonstrate their commitment to safety. This trend is expected to contribute to the market's expansion, as companies recognize the importance of quality control in sustaining their reputation and competitiveness.