Global Supply Chain Challenges
The US Pharmaceutical Quality Control Market is currently navigating global supply chain challenges that impact quality control processes. Disruptions in the supply chain can lead to inconsistencies in raw materials, which in turn affect the quality of pharmaceutical products. As a response, companies are increasingly investing in quality control measures to mitigate risks associated with supply chain vulnerabilities. In 2025, it is anticipated that approximately 25% of pharmaceutical companies in the US will enhance their quality control protocols to address these challenges. This proactive approach is likely to foster growth in the US Pharmaceutical Quality Control Market as companies seek to ensure the integrity of their products amidst global uncertainties.
Stringent Regulatory Frameworks
The US Pharmaceutical Quality Control Market is significantly influenced by stringent regulatory frameworks established by the FDA and other governing bodies. These regulations mandate rigorous testing and validation processes to ensure the safety and efficacy of pharmaceutical products. In recent years, the FDA has increased its focus on compliance, resulting in a higher number of inspections and enforcement actions. This regulatory environment compels pharmaceutical companies to enhance their quality control measures, leading to increased investments in quality assurance technologies. As a result, the US Pharmaceutical Quality Control Market is expected to expand as companies strive to meet these evolving regulatory requirements.
Increased Focus on Patient Safety
The US Pharmaceutical Quality Control Market is increasingly prioritizing patient safety, which is becoming a central tenet of pharmaceutical manufacturing. With rising public awareness regarding drug safety and efficacy, pharmaceutical companies are compelled to adopt stringent quality control measures to protect consumers. The FDA's initiatives to enhance drug safety standards have led to a greater emphasis on quality assurance throughout the production process. In 2025, it is projected that investments in quality control systems aimed at ensuring patient safety will account for a significant portion of the pharmaceutical industry's budget. This focus on patient safety is likely to drive the growth of the US Pharmaceutical Quality Control Market as companies strive to maintain consumer trust.
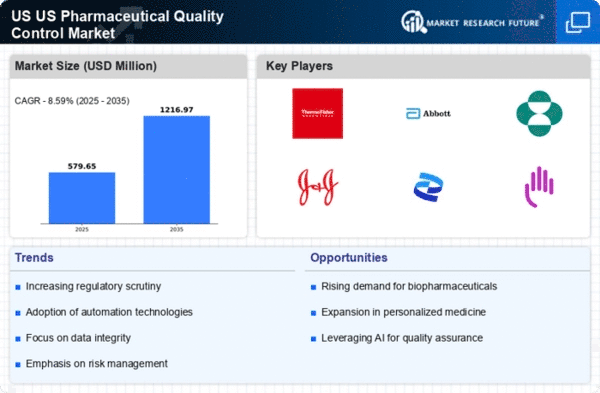
Rising Demand for Biopharmaceuticals
The US Pharmaceutical Quality Control Market is experiencing a notable surge in demand for biopharmaceuticals, driven by advancements in biotechnology and personalized medicine. As biopharmaceuticals often require stringent quality control measures, the industry is adapting to ensure compliance with regulatory standards. In 2025, the biopharmaceutical sector accounted for approximately 40% of the total pharmaceutical market in the US, highlighting the necessity for robust quality control systems. This trend is likely to continue, as the FDA emphasizes the importance of quality assurance in biopharmaceutical production. Consequently, companies are investing in sophisticated quality control technologies to meet these demands, thereby propelling growth in the US Pharmaceutical Quality Control Market.
Technological Advancements in Quality Control
The US Pharmaceutical Quality Control Market is witnessing a transformation due to technological advancements in quality control processes. Innovations such as automation, artificial intelligence, and data analytics are being integrated into quality assurance protocols, enhancing efficiency and accuracy. For instance, the adoption of real-time monitoring systems allows for immediate detection of deviations in production processes, thereby reducing the risk of non-compliance. In 2025, it is estimated that over 30% of pharmaceutical companies in the US have implemented advanced quality control technologies, indicating a shift towards more proactive quality management. This trend is likely to drive growth in the US Pharmaceutical Quality Control Market as companies seek to leverage technology for improved quality outcomes.