Rising Incidence of Cancer
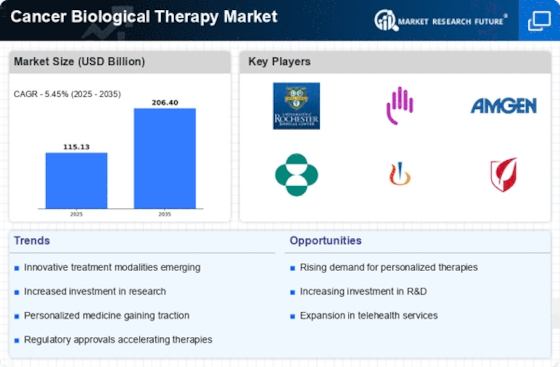
The increasing prevalence of cancer worldwide is a primary driver for the Cancer Biological Therapy Market. According to recent statistics, cancer cases are projected to rise significantly, with estimates suggesting that by 2040, the number of new cancer cases could reach over 27 million annually. This alarming trend necessitates the development of innovative treatment options, including biological therapies, which are designed to target specific cancer cells while minimizing damage to healthy tissues. As healthcare systems strive to address this growing burden, investments in research and development of biological therapies are likely to surge, thereby propelling the Cancer Biological Therapy Market forward.
Advancements in Biotechnology
Technological advancements in biotechnology are transforming the landscape of the Cancer Biological Therapy Market. Innovations such as CRISPR gene editing, monoclonal antibodies, and CAR T-cell therapy are revolutionizing treatment paradigms. For instance, the market for monoclonal antibodies alone is expected to exceed USD 100 billion by 2025, reflecting the increasing reliance on targeted therapies. These advancements not only enhance the efficacy of treatments but also improve patient outcomes, leading to a growing demand for biological therapies. As research continues to unveil new therapeutic targets, the Cancer Biological Therapy Market is poised for substantial growth.
Increased Investment in Cancer Research
The Cancer Biological Therapy Market is experiencing a surge in investment from both public and private sectors. Governments and organizations are allocating substantial funds to cancer research initiatives, with global spending on cancer research estimated to reach USD 200 billion by 2025. This influx of capital is facilitating the development of novel biological therapies and fostering collaborations between academic institutions and pharmaceutical companies. As a result, the Cancer Biological Therapy Market is likely to benefit from accelerated innovation and a broader pipeline of therapeutic options, ultimately enhancing treatment accessibility for patients.
Growing Awareness and Screening Programs
Rising awareness about cancer and the importance of early detection is driving the Cancer Biological Therapy Market. Public health campaigns and screening programs are becoming increasingly prevalent, leading to earlier diagnoses and a higher demand for effective treatment options. As more individuals are diagnosed at earlier stages, the need for advanced therapies, including biological treatments, is expected to grow. This trend is further supported by the increasing emphasis on personalized medicine, which tailors treatment to individual patient profiles. Consequently, the Cancer Biological Therapy Market is likely to expand as healthcare providers seek to offer the most effective therapies available.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supportive of innovative therapies, which is positively impacting the Cancer Biological Therapy Market. Initiatives such as accelerated approval pathways and orphan drug designations are designed to expedite the development and availability of new treatments. For example, the FDA has implemented programs that allow for faster review of breakthrough therapies, which can significantly shorten the time from research to market. This regulatory environment encourages pharmaceutical companies to invest in the development of biological therapies, thereby enhancing the diversity of treatment options available to patients. As a result, the Cancer Biological Therapy Market is likely to witness robust growth in the coming years.