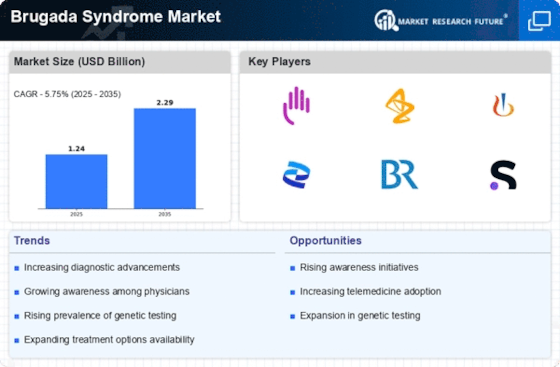
Top Industry Leaders in the Brugada Syndrome Market

Latest Brugada Syndrome Companies Update
Amarin Corporation Phase 3 ELECTRA study evaluating the investigational drug, VASCEPA® (icosapent ethyl), in preventing major adverse cardiovascular events (MACE) in Brugada Syndrome patients with a history of syncope or aborted cardiac arrest is ongoing. Positive results could significantly improve outcomes for this high-risk patient population.
Taisho Pharmaceutical Holdings Co. Phase 3 BRAVO study assessing ranolazine for preventing ventricular fibrillation (VF) in Brugada Syndrome patients is nearing completion. Ranolazine's potential to address the electrical instability characteristic of Brugada Syndrome makes this trial highly anticipated.
Pfizer pharmaceutical giant is actively involved in research collaborations to advance the understanding and treatment of Brugada Syndrome. They recently partnered with the Brugada Syndrome Foundation to support a patient registry and research initiatives.
Abbott Laboratories is focusing on developing minimally invasive devices for Brugada Syndrome management. Their Farapulse® catheter ablation system provides a potentially safer and more effective alternative to traditional surgical procedures.
List of Brugada Syndrome Key Companies in the Market
-
GeneDx (U.S)
-
PGxHealth LLC (U.S)
-
Abbott (U.S)
-
GE Healthcare (U.K)
-
Medtronic (U.S.)
-
Pfizer, Inc (U.S)
-
Boston Scientific Corporation (U.S.)
-
GlaxoSmithKline (U.K)
-
Eli Lilly Company (U.S)
-
Taj Pharmaceuticals Ltd (India)