Growing Incidence of Genetic Testing
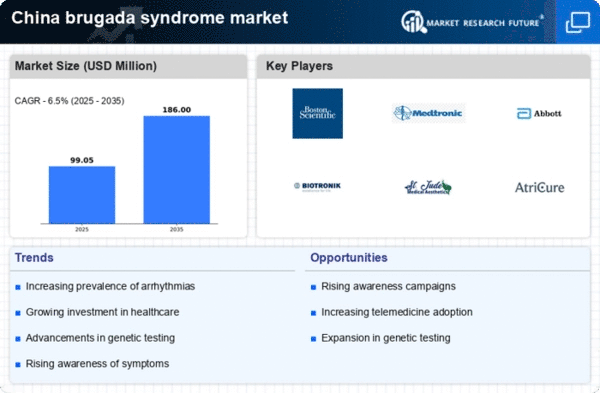
The growing incidence of genetic testing for inherited cardiac conditions is a notable driver for the brugada syndrome market. In China, advancements in genetic testing technologies have made it more accessible and affordable for patients. As more individuals undergo genetic testing, the identification of brugada syndrome cases is expected to rise, leading to increased demand for specialized care and management. This trend is particularly relevant given the hereditary nature of the syndrome, which necessitates family screening and counseling. Consequently, the market may experience growth as healthcare providers seek to offer comprehensive genetic services alongside traditional diagnostic methods.
Increasing Awareness of Brugada Syndrome
The rising awareness of Brugada syndrome among healthcare professionals and the general public is a crucial driver for the brugada syndrome market. Educational campaigns and initiatives by health organizations in China have contributed to a better understanding of the condition, which is characterized by specific ECG patterns and an increased risk of sudden cardiac death. As awareness grows, more individuals are likely to seek medical advice and diagnostic testing, leading to an increase in demand for related healthcare services and products. This heightened awareness is expected to drive market growth, as it encourages early diagnosis and intervention, potentially reducing mortality rates associated with the syndrome.
Regulatory Support for Innovative Treatments
Regulatory bodies in China are increasingly supportive of innovative treatments for rare diseases, including brugada syndrome. This support is reflected in streamlined approval processes for new therapies and diagnostic tools, which can significantly impact the brugada syndrome market. The Chinese government has implemented policies to encourage research and development in the field of cardiology, which may lead to the introduction of novel therapies and devices. As a result, The market could see a surge in new product offerings. This would enhance treatment options for patients and potentially improve outcomes.. This regulatory environment fosters a conducive atmosphere for market expansion.
Expansion of Cardiology Services in Rural Areas
The expansion of cardiology services in rural China significantly drives the brugada syndrome market.. Historically, access to specialized cardiac care has been limited in these regions, leading to underdiagnosis and undertreatment of conditions like brugada syndrome. However, recent initiatives aimed at improving healthcare infrastructure and training healthcare professionals in rural settings are changing this landscape. As cardiology services become more accessible, it is anticipated that more patients will receive timely diagnoses and appropriate management for brugada syndrome. This increased access could lead to a broader market reach and enhanced patient outcomes.
Technological Advancements in Cardiac Monitoring
Technological advancements in cardiac monitoring devices are transforming the landscape of the brugada syndrome market. Innovations such as wearable ECG monitors and remote patient monitoring systems are becoming increasingly prevalent in China. These devices allow for continuous monitoring of patients at risk of arrhythmias, facilitating timely interventions. The integration of artificial intelligence in these technologies enhances their predictive capabilities, potentially improving patient outcomes. As healthcare providers adopt these advanced monitoring solutions, the demand for related products and services in the brugada syndrome market is likely to increase, reflecting a shift towards proactive management of cardiac health.