-
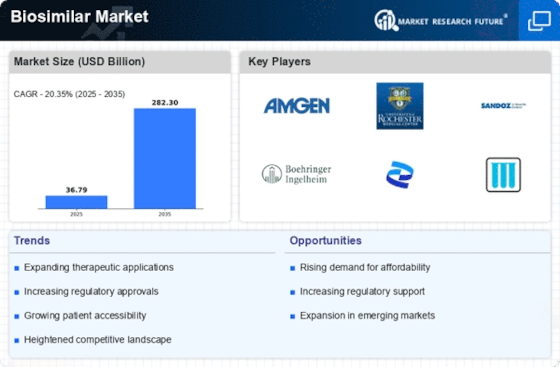
EXECUTIVE SUMMARY
-
MARKET INTRODUCTION
-
DEFINITION
-
SCOPE OF THE STUDY
-
RESEARCH OBJECTIVE
-
MARKET STRUCTURE
-
RESEARCH METHODOLOGY
-
OVERVIEW
-
DATA FLOW
- DATA MINING PROCESS
-
PURCHASED DATABASE:
-
SECONDARY SOURCES:
- SECONDARY RESEARCH DATA FLOW:
-
PRIMARY RESEARCH:
- PRIMARY RESEARCH DATA FLOW:
- PRIMARY RESEARCH: NUMBER OF INTERVIEWS CONDUCTED
- PRIMARY RESEARCH: REGIONAL COVERAGE
-
APPROACHES FOR MARKET SIZE ESTIMATION:
- REVENUE ANALYSIS APPROACH
-
DATA FORECASTING
- DATA FORECASTING TYPE
-
DATA MODELING
- MICROECONOMIC FACTOR ANALYSIS:
- DATA MODELING:
-
TEAMS AND ANALYST CONTRIBUTION
-
MARKET DYNAMICS
-
INTRODUCTION
-
DRIVERS
- GROWING INCIDENCES OF CANCER AND RARE DISORDERS
- INCREASING LAUNCH OF BIOSIMILARS
- RISING HEALTHCARE COSTS
-
RESTRAINTS
- REGULATORY AND APPROVAL BARRIERS
- LIMITED REIMBURSEMENT AND MARKET ACCESS
-
OPPORTUNITY
- EXPANSION IN EMERGING MARKETS
- PARTNERSHIPS AND COLLABORATIONS
-
MARKET FACTOR ANALYSIS
-
PORTER'S FIVE FORCES MODEL
- THREAT OF NEW ENTRANTS
- BARGAINING POWER OF SUPPLIERS
- THREAT OF SUBSTITUTES
- BARGAINING POWER OF BUYERS
- INTENSITY OF RIVALRY
-
IMPACT OF COVID-19 ON GLOBAL BIOSIMILAR MARKET
-
GLOBAL BIOSIMILAR MARKET, BY DRUG CLASS
-
OVERVIEW
-
MONOCLONAL ANTIBODIES
- ADALIMUMAB
- INFLIXIMAB
- RITUXIMAB
- BEVACIZUMAB
- TRASTUZUMAB
- USTEKINUMAB
- TOCILIZUMAB
- AFLIBERCEPT
- DUPILUMAB
- DENOSUMAB
- OTHERS
-
INSULIN
-
GRANULOCYTE COLONY- STIMULATING FACTOR
-
ERYTHROPOIETIN
-
RECOMBINANT HUMAN GROWTH HORMONE
-
ETANERCEPT
-
FOLLITROPIN
-
TERIPARATIDE
-
ANTICOAGULANTS
-
OTHERS
-
GLOBAL BIOSIMILAR MARKET, BY APPLICATION
-
OVERVIEW
-
ONCOLOGY
- BREAST CANCER
- LUNG CANCER
- PROSTATE CANCER
- LEUKEMIA
- BLADDER CANCER
- COLORECTAL CANCER
- OTHERS
-
AUTOIMMUNE DISEASES
-
INFECTIOUS DISEASES
-
BLOOD DISORDERS
-
OTHERS
-
GLOBAL BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION
-
OVERVIEW
-
SUBCUTANEOUS
-
INTRAVENOUS
-
GLOBAL BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL
-
OVERVIEW
-
HOSPITAL PHARMACIES
-
RETAIL PHARMACIES
-
ONLINE PHARMACIES
-
SPECIALTY PHARMACIES
-
GLOBAL BIOSIMILAR MARKET, BY REGION
-
OVERVIEW
-
NORTH AMERICA
- US
- CANADA
- MEXICO
-
EUROPE
- GERMANY
- FRANCE
- UK
- ITALY
- SPAIN
- REST OF EUROPE
-
ASIA-PACIFIC
- CHINA
- INDIA
- JAPAN
- AUSTRALIA
- SOUTH KOREA
- REST OF ASIA-PACIFIC
-
REST OF THE WORLD
- MIDDLE EAST AND AFRICA
- SOUTH AMERICA
-
COMPETITIVE LANDSCAPE
-
INTRODUCTION
-
MARKET SHARE ANALYSIS, 2024
-
COMPETITOR DASHBOARD
-
PUBLIC PLAYERS STOCK SUMMARY
-
COMPARATIVE ANALYSIS: KEY PLAYERS FINANCIAL
-
KEY DEVELOPMENTS & GROWTH STRATEGIES
- PRODUCT LAUNCH
- PRODUCT APPROVAL
- AGREEMENT/COLLABORATION/PARTNERSHIP
-
COMPANY PROFILE
-
ELI LILLY AND COMPANY
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
TEVA PHARMACEUTICAL INDUSTRIES LTD.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
SAMSUNG BIOEPIS
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
AMGEN INC.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
PFIZER INC.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
NOVARTIS AG
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
BIOGEN
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
BIOCON
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
DR. REDDY’S LABORATORIES LTD.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCT OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
FRESENIUS KABI USA, LLC.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
KIDSWELL BIO CORPORATION
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCT OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
DATA CITATIONS
-
DATA CITATIONS
-
DATA CITATIONS
-
-
LIST OF TABLES
-
QFD MODELING FOR MARKET SHARE ASSESSMENT
-
GLOBAL BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR ADALIMUMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR INFLIXIMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR RITUXIMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR BEVACIZUMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR TRASTUZUMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR USTEKINUMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR TOCILIZUMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR AFLIBERCEPT, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR DUPILUMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR DENOSUMAB, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR OTHERS, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR INSULIN, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR GRANULOCYTE COLONY- STIMULATING FACTOR, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR ERYTHROPOIETIN, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR RECOMBINANT HUMAN GROWTH HORMONE, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR ETANERCEPT, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR FOLLITROPIN, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR TERIPARATIDE, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR ANTICOAGULANTS, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR OTHERS, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR ONCOLOGY, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR BREAST CANCER, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR LUNG CANCER, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR PROSTATE CANCER, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR LEUKEMIA, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR BLADDER CANCER, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR COLORECTAL CANCER, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR OTHERS, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR AUTOIMMUNE DISEASES, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR INFECTIOUS DISEASES, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR BLOOD DISORDERS, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR OTHERS, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR SUBCUTANEOUS, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR INTRAVENOUS, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR HOSPITAL PHARMACIES, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR RETAIL PHARMACIES, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR ONLINE PHARMACIES, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, FOR SPECIALTY PHARMACIES, BY REGION, 2019–2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, BY REGION, 2019-2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET, BY COUNTRY, 2019-2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
US BIOSIMILAR MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
US BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
US BIOSIMILAR MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
US BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
US BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
US BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
CANADA BIOSIMILAR MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
CANADA BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
CANADA BIOSIMILAR MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
CANADA BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
CANADA BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
CANADA BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
MEXICO BIOSIMILAR MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
MEXICO BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
MEXICO BIOSIMILAR MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
MEXICO BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
MEXICO BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
MEXICO BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET, BY COUNTRY, 2019-2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET, FOR MONICLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
GERMANY BIOSIMILARS MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
GERMANY BIOSIMILARS MARKET, FOR MONICLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
GERMANY BIOSIMILARS MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
GERMANY BIOSIMILARS MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
GERMANY BIOSIMILARS MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
GERMANY BIOSIMILARS MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
FRANCE BIOSIMILARS MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
FRANCE BIOSIMILARS MARKET, FOR MONICLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
FRANCE BIOSIMILARS MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
FRANCE BIOSIMILARS MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
FRANCE BIOSIMILARS MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
FRANCE BIOSIMILARS MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
UK BIOSIMILARS MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
UK BIOSIMILARS MARKET, FOR MONICLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
UK BIOSIMILARS MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
UK BIOSIMILARS MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
UK BIOSIMILARS MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
UK BIOSIMILARS MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
ITALY BIOSIMILARS MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
ITALY BIOSIMILARS MARKET, FOR MONICLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
ITALY BIOSIMILARS MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
ITALY BIOSIMILARS MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
ITALY BIOSIMILARS MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
ITALY BIOSIMILARS MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
SPAIN BIOSIMILARS MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
SPAIN BIOSIMILARS MARKET, FOR MONICLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
SPAIN BIOSIMILARS MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
SPAIN BIOSIMILARS MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
SPAIN BIOSIMILARS MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
SPAIN BIOSIMILARS MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
REST OF EUROPE BIOSIMILARS MARKET, BY DRUG CLASS, 2019-2035 (USD BILLION)
-
REST OF EUROPE BIOSIMILARS MARKET, FOR MONICLONAL ANTIBODIES, BY TYPE, 2019-2035 (USD BILLION)
-
REST OF EUROPE BIOSIMILARS MARKET, BY APPLICATION, 2019-2035 (USD BILLION)
-
REST OF EUROPE BIOSIMILARS MARKET, FOR ONCOLOGY, BY TYPE, 2019-2035 (USD BILLION)
-
REST OF EUROPE BIOSIMILARS MARKET, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD BILLION)
-
REST OF EUROPE BIOSIMILARS MARKET, BY DISTRIBUTION CHANNEL, 2019-2035 (USD BILLION)
-
ASIA-PACIFIC: BIOSIMILAR MARKET, BY COUNTRY, 2019–2035 (USD BILLION)
-
ASIA-PACIFIC: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
ASIA-PACIFIC: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
ASIA-PACIFIC: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
ASIA-PACIFIC: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
ASIA-PACIFIC: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
ASIA-PACIFIC: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
CHINA: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
CHINA: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
CHINA: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
CHINA: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
CHINA: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
CHINA: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
INDIA -PACIFIC: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
INDIA: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
INDIA: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
INDIA: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
INDIA: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
INDIA: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
JAPAN: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
JAPAN: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
JAPAN: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
JAPAN: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
JAPAN: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
JAPAN: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
AUSTRALIA: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
AUSTRALIA: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
AUSTRALIA: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
AUSTRALIA: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
AUSTRALIA: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
AUSTRALIA: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
SOUTH KOREA: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
SOUTH KOREA: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
SOUTH KOREA: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
SOUTH KOREA: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
SOUTH KOREA: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
SOUTH KOREA: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
REST OF ASIA-PACIFIC: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
REST OF ASIA-PACIFIC: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
REST OF ASIA-PACIFIC: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
REST OF ASIA-PACIFIC: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
REST OF ASIA-PACIFIC: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
REST OF ASIA-PACIFIC: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
REST OF THE WORLD: BIOSIMILAR MARKET, BY COUNTRY, 2019–2035 (USD BILLION)
-
REST OF THE WORLD: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
REST OF THE WORLD: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
REST OF THE WORLD: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
REST OF THE WORLD: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
REST OF THE WORLD: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
REST OF THE WORLD: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
MIDDLE EAST AND AFRICA: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
MIDDLE EAST AND AFRICA: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
MIDDLE EAST AND AFRICA: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
MIDDLE EAST AND AFRICA: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
MIDDLE EAST AND AFRICA: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
MIDDLE EAST AND AFRICA: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
SOUTH AMERICA: BIOSIMILAR MARKET, BY DRUG CLASS, 2019–2035 (USD BILLION)
-
SOUTH AMERICA: BIOSIMILAR MARKET, FOR MONOCLONAL ANTIBODIES, BY TYPE, 2019–2035 (USD BILLION)
-
SOUTH AMERICA: BIOSIMILAR MARKET, BY APPLICATION, 2019–2035 (USD BILLION)
-
SOUTH AMERICA: BIOSIMILAR MARKET, FOR ONCOLOGY, BY TYPE, 2019–2035 (USD BILLION)
-
SOUTH AMERICA: BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2019–2035 (USD BILLION)
-
SOUTH AMERICA: BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2019–2035 (USD BILLION)
-
PUBLIC PLAYERS STOCK SUMMARY
-
PRODUCT LAUNCH
-
PRODUCT APPROVAL
-
AGREEMENT/COLLABORATION/PARTNERSHIP
-
ELI LILLY AND COMPANY: PRODUCTS OFFERED
-
ELI LILLY AND COMPANY: KEY DEVELOPMENTS
-
TEVA PHARMACEUTICAL INDUSTRIES LTD.: PRODUCTS OFFERED
-
TEVA PHARMACEUTICAL INDUSTRIES LTD.: KEY DEVELOPMENTS
-
SAMSUNG BIOEPIS: PRODUCT OFFERED
-
SAMSUNG BIOEPIS: KEY DEVELOPMENTS
-
AMGEN INC.: PRODUCTS OFFERED
-
AMGEN INC.: KEY DEVELOPMENTS
-
PFIZER INC.: PRODUCTS OFFERED
-
PFIZER INC.: KEY DEVELOPMENTS
-
NOVARTIS AG: PRODUCTS OFFERED
-
NOVARTIS AG: KEY DEVELOPMENTS
-
BIOGEN: PRODUCTS OFFERED
-
BIOGEN: KEY DEVELOPMENTS
-
BIOCON: PRODUCTS OFFERED
-
BIOCON: KEY DEVELOPMENTS
-
DR. REDDY'S LABORATORIES LTD.: PRODUCTS OFFERED
-
DR. REDDY LABORATORIES: KEY DEVELOPMENTS
-
FRESENIUS KABI USA, LLC.: PRODUCT OFFERED
-
FRESENIUS KABI USA, LLC.: KEY DEVELOPMENTS
-
KIDSWELL BIO CORPORATION: PRODUCTS OFFERED
-
PROCTER & GAMBLE: KEY DEVELOPMENTS
-
-
LIST OF FIGURES
-
GLOBAL BIOSIMILARS MARKET: STRUCTURE
-
GLOBAL BIOSIMILAR MARKET: MARKET GROWTH FACTOR ANALYSIS (2024-2035)
-
DRIVER IMPACT ANALYSIS (2024-2035)
-
RESTRAINT IMPACT ANALYSIS (2024-2035)
-
PORTER'S FIVE FORCES ANALYSIS: GLOBAL BIOSIMILAR MARKET
-
GLOBAL BIOSIMILAR MARKET, BY DRUG CLASS, SEGMENT ATTRACTIVENESS ANALYSIS
-
GLOBAL BIOSIMILAR MARKET, MONOCLONAL ANTIBODIES, BY TYPE SEGMENT ATTRACTIVENESS, 2024 & 2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET, BY DRUG CLASS, 2024 & 2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET SHARE (%), BY DRUG CLASS, 2024
-
GLOBAL BIOSIMILAR MARKET, BY APPLICATION, SEGMENT ATTRACTIVENESS ANALYSIS
-
GLOBAL BIOSIMILAR MARKET, BY APPLICATION, 2024 & 2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET SHARE (%), BY APPLICATION, 2024
-
GLOBAL BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, SEGMENT ATTRACTIVENESS ANALYSIS
-
GLOBAL BIOSIMILAR MARKET, BY ROUTE OF ADMINISTRATION, 2024 & 2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET SHARE (%), BY APPLICATION, 2024
-
GLOBAL BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, SEGMENT ATTRACTIVENESS ANALYSIS
-
GLOBAL BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL, 2024 & 2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET SHARE (%), BY DISTRIBUTION CHANNEL, 2024
-
GLOBAL BIOSIMILAR MARKET, BY REGION, 2024 & 2035 (USD BILLION)
-
GLOBAL BIOSIMILAR MARKET SHARE (%), BY REGION, 2024
-
NORTH AMERICA MARKET ANALYSIS: BIOSIMILAR MARKET, 2019-2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET, BY COUNTRY, 2024 & 2035 (USD BILLION)
-
NORTH AMERICA BIOSIMILAR MARKET SHARE (%), BY COUNTRY, 2024
-
EUROPE MARKET ANALYSIS: BIOSIMILARS MARKET, 2019-2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET, BY COUNTRY, 2024 & 2035 (USD BILLION)
-
EUROPE BIOSIMILARS MARKET SHARE (%), BY COUNTRY, 2024
-
ASIA-PACIFIC: BIOSIMILAR MARKET SHARE, BY COUNTRY, 2024 & 2035 (USD BILLION)
-
ASIA-PACIFIC: BIOSIMILAR MARKET SHARE, BY COUNTRY, 2024 (%)
-
REST OF THE WORLD: BIOSIMILAR MARKET SHARE, BY COUNTRY, 2024 & 2035 (USD BILLION)
-
REST OF THE WORLD: BIOSIMILAR MARKET SHARE, BY COUNTRY, 2024 (%)
-
GLOBAL BIOSIMILAR MARKET PLAYERS: COMPETITIVE ANALYSIS, 2024
-
COMPETITOR DASHBOARD: GLOBAL BIOSIMILAR MARKET
-
ELI LILLY AND COMPANY: FINANCIAL OVERVIEW SNAPSHOT
-
ELI LILLY AND COMPANY: SWOT ANALYSIS
-
TEVA PHARMACEUTICAL INDUSTRIES LTD.: FINANCIAL OVERVIEW SNAPSHOT
-
TEVA PHARMACEUTICAL INDUSTRIES LTD.: SWOT ANALYSIS
-
AMGEN INC.: FINANCIAL OVERVIEW SNAPSHOT
-
AMGEN INC.: SWOT ANALYSIS
-
PFIZER INC.: FINANCIAL OVERVIEW SNAPSHOT
-
PFIZER INC.: SWOT ANALYSIS
-
NOVARTIS AG: FINANCIAL OVERVIEW SNAPSHOT
-
BIOGEN: FINANCIAL OVERVIEW SNAPSHOT
-
BIOCON: FINANCIAL OVERVIEW SNAPSHOT
-
BIOCON.: SWOT ANALYSIS
-
DR. REDDY'S LABORATORIES LTD.: FINANCIAL OVERVIEW SNAPSHOT
-
KIDSWELL BIO CORPORATION: FINANCIAL OVERVIEW SNAPSHOT
-
KIDSWELL BIO CORPORATION: SWOT ANALYSIS
-
"
















Leave a Comment