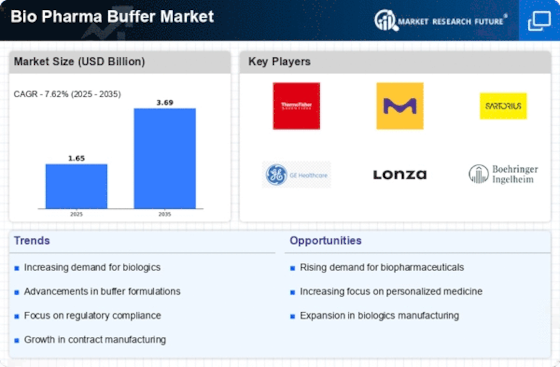
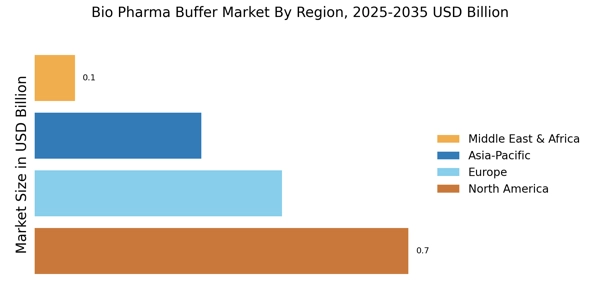
Growing Focus on Biosimilars
The Bio Pharma Buffer Market is benefiting from the rising focus on biosimilars, which are biologic medical products highly similar to already approved reference products. As healthcare systems seek cost-effective alternatives to expensive biologics, the demand for biosimilars is projected to grow significantly. In 2025, the biosimilars market is anticipated to reach a valuation of approximately 100 billion USD. This growth necessitates the use of specialized buffer solutions during the development and production of biosimilars, as they require precise formulation to ensure efficacy and safety. Consequently, the increasing emphasis on biosimilars is likely to drive demand within the Bio Pharma Buffer Market, as manufacturers seek to optimize their production processes.
Emerging Markets and Globalization
The Bio Pharma Buffer Market is experiencing growth due to the emergence of new markets and the globalization of biopharmaceutical production. Countries in Asia and Latin America are increasingly becoming hubs for biopharmaceutical manufacturing, driven by lower production costs and favorable regulatory environments. This shift is expected to create new opportunities for buffer solution providers as these regions ramp up their production capabilities. In 2025, it is projected that the biopharmaceutical market in these emerging regions will grow at a rate of 10% annually. As a result, the demand for high-quality buffers tailored to local manufacturing needs is likely to rise, further propelling the Bio Pharma Buffer Market.
Expansion of Biomanufacturing Facilities
The Bio Pharma Buffer Market is poised for growth due to the expansion of biomanufacturing facilities worldwide. As the demand for biopharmaceuticals escalates, companies are investing in new production sites to enhance their manufacturing capabilities. In 2025, it is estimated that the biomanufacturing sector will witness a compound annual growth rate of around 8%, leading to an increased need for buffer solutions that facilitate efficient production processes. These facilities require buffers that can withstand varying conditions and maintain stability during large-scale production. Thus, the expansion of biomanufacturing is expected to create substantial opportunities for the Bio Pharma Buffer Market, as manufacturers seek reliable buffer solutions to support their operations.
Increasing Investment in Biopharma Research
The Bio Pharma Buffer Market is experiencing a surge in investment as pharmaceutical companies allocate substantial resources towards biopharmaceutical research and development. This trend is driven by the growing need for innovative therapies and personalized medicine. In 2025, the biopharmaceutical sector is projected to reach a market value of approximately 500 billion USD, indicating a robust growth trajectory. As research intensifies, the demand for high-quality buffer solutions, essential for maintaining pH and ionic strength during bioprocessing, is likely to increase. Consequently, this investment trend is expected to bolster the Bio Pharma Buffer Market, as companies seek reliable and efficient buffer solutions to support their research initiatives.
Regulatory Compliance and Quality Standards
The Bio Pharma Buffer Market is significantly influenced by stringent regulatory requirements and quality standards imposed by health authorities. These regulations ensure that biopharmaceutical products meet safety and efficacy benchmarks, necessitating the use of high-quality buffer solutions in manufacturing processes. As regulatory bodies continue to evolve their guidelines, companies are compelled to adopt advanced buffer formulations that comply with these standards. This compliance not only enhances product quality but also mitigates risks associated with regulatory non-compliance. The increasing focus on quality assurance in biopharmaceutical manufacturing is likely to drive demand for specialized buffers, thereby positively impacting the Bio Pharma Buffer Market.