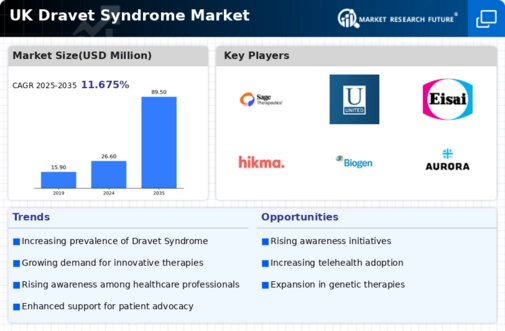
The UK Dravet Syndrome Market has experienced significant growth and development in recent years, shaped by a combination of innovative drug therapies, enhanced research initiatives, and increased awareness surrounding this rare form of epilepsy. As more stakeholders engage with this niche market, a clearer picture of the competitive landscape has emerged.
This involves not only understanding the existing players but also assessing market strategies, identifying gaps for potential entrants, and recognizing the dynamics that drive demand for effective treatments.
The complexities of the market are further heightened by the regulatory landscape, which governs the approval and management of treatments for Dravet Syndrome in the UK, and the evolving needs of patients, caregivers, and healthcare providers.
Insolia Therapeutics has established a solid presence in the UK Dravet Syndrome Market, bolstered by its strong research and development capabilities, focused commitment to addressing unmet medical needs, and established relationships with healthcare providers. The company’s strength lies in its dedicated approach to developing targeted therapies designed specifically for patients with Dravet Syndrome, emphasizing efficacy, safety, and accessibility.
By concentrating its efforts on this specific condition, Insolia Therapeutics is positioned well to meet the demands of a unique patient population. Furthermore, its investment in local partnerships and collaborations within the UK healthcare ecosystem enhances its credibility, providing the company with an in-depth understanding of market dynamics and opportunities for growth in the region.
Sage Therapeutics, while also making its mark in the UK Dravet Syndrome Market, has focused on delivering innovative treatment solutions through a pipeline of products designed for neurological conditions.
The company's key products often leverage novel mechanisms of action to provide safer and more effective treatment options. Sage Therapeutics’ strength in the market lies in its robust clinical trial programs and a commitment to addressing the specific needs of Dravet Syndrome patients in the UK. With strategic mergers and acquisitions aimed at expanding its portfolio and enhancing its research capabilities, Sage Therapeutics continues to position itself as a significant player in the UK market.
Its ongoing collaborations with local healthcare institutions further solidify its presence, allowing the company to remain agile and responsive to the nuances of the UK healthcare landscape. This proactive engagement helps to ensure that Sage Therapeutics can adapt to evolving patient needs and regulatory challenges within the UK Dravet Syndrome Market.