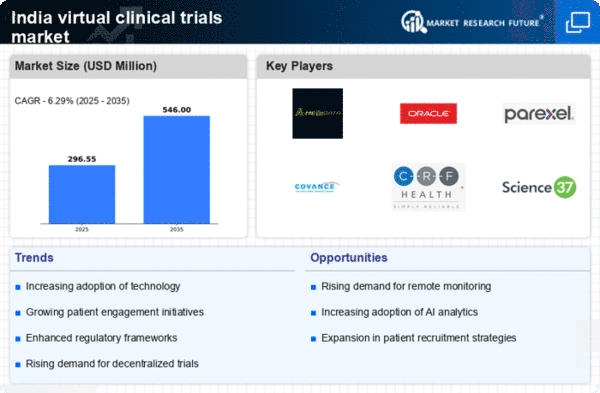
The India Virtual Clinical Trials Market has seen a remarkable evolution over the past few years, driven by technological advancements, regulatory support, and increasing demand for cost-effective, efficient trial methodologies. This market offers a unique platform for conducting clinical research remotely, which addresses challenges such as patient recruitment and geographical limitations. The competitive landscape in this sector is characterized by a mix of established firms and new players striving to leverage digital health technologies, artificial intelligence, and data analytics to enhance trial efficiency and participant engagement.
As the sector matures, companies are increasingly focusing on shaping their strategies to capture market share, improve operational efficiencies, and enhance the patient experience, all while responding to the dynamic needs of stakeholders involved in the clinical trial ecosystem.
Parexel International has established a strong foothold in the India Virtual Clinical Trials Market, leveraging its extensive experience in the clinical research industry. The company is noted for its robust operational capabilities and a comprehensive suite of end-to-end solutions tailored for virtual and hybrid clinical trials. Parexel's strengths lie in its ability to integrate technology with clinical research expertise, thus enabling streamlined patient recruitment, real-time data monitoring, and enhanced patient retention. Additionally, the company’s vast global network provides an edge in reaching diverse patient populations across India, which is essential for the success of virtual clinical trials.
Parexel's commitment to innovation and adherence to regulatory standards exemplify its reputation as a trusted partner in this competitive market segment.
Covance, as a prominent player in the India Virtual Clinical Trials Market, is recognized for its extensive portfolio of services, which includes drug development services, regulatory consulting, and comprehensive clinical trial management solutions. The company capitalizes on cutting-edge technology to offer virtual trial capabilities, ensuring that it meets the needs of sponsors and patients alike. Covance's strengths encompass a strong operational infrastructure in India that supports efficient trial execution and patient-centric approaches tailored to regional demographics. Notably, the firm has actively sought out opportunities for strategic alliances and acquisitions to expand its service offerings and enhance its market presence.
Through ongoing initiatives to innovate and implement advanced data analytics in clinical trials, Covance has solidified its position as a leader in the virtual space, demonstrating its ability to adapt and thrive within the evolving landscape of clinical research in India.