Regulatory Evolution and Adaptation
The evolving regulatory landscape in India is playing a pivotal role in shaping the virtual clinical-trials market. Regulatory bodies are increasingly recognizing the importance of virtual trials and are adapting guidelines to facilitate their implementation. By 2025, it is expected that new regulations will streamline the approval process for virtual trials, potentially reducing timelines by up to 30%. This regulatory support is crucial for fostering innovation and encouraging more sponsors to adopt virtual methodologies. As the virtual clinical-trials market continues to align with these evolving regulations, it is likely to witness accelerated growth and increased participation from various stakeholders.
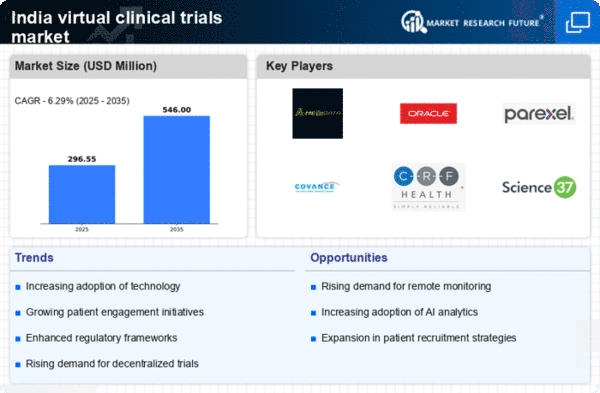
Rising Demand for Decentralized Trials
The virtual clinical-trials market in India is experiencing a notable increase in demand for decentralized trials. This shift is largely driven by the need for more patient-centric approaches, allowing participants to engage in trials from their homes. As of 2025, it is estimated that around 60% of clinical trials in India are adopting decentralized methodologies. This trend not only enhances patient recruitment but also improves retention rates, as participants find it more convenient to participate without the need for frequent hospital visits. The virtual clinical-trials market is thus evolving to meet these demands, potentially leading to faster trial completion times and reduced costs for sponsors.
Growing Patient Awareness and Engagement
Patient awareness and engagement are emerging as vital drivers for the virtual clinical-trials market in India. As patients become more informed about clinical research and its benefits, their willingness to participate in trials is increasing. Surveys indicate that around 70% of patients are now more open to participating in virtual trials compared to traditional ones. This heightened awareness is fostering a more engaged patient population, which is essential for the success of clinical trials. Consequently, the virtual clinical-trials market is likely to benefit from this trend, as increased patient participation can lead to more diverse and representative trial populations.
Advancements in Digital Health Technologies
The integration of digital health technologies is significantly influencing the virtual clinical-trials market in India. Innovations such as wearable devices, mobile health applications, and telemedicine platforms are facilitating real-time data collection and patient monitoring. By 2025, it is projected that the use of digital tools in clinical trials will increase by approximately 40%. These advancements not only streamline the data collection process but also enhance the overall efficiency of trials. Consequently, the virtual clinical-trials market is likely to see a surge in the adoption of these technologies, which could lead to improved patient outcomes and more robust trial results.
Increased Investment in Research and Development
Investment in research and development (R&D) is a critical driver for the virtual clinical-trials market in India. With the government and private sectors allocating substantial funds towards innovative healthcare solutions, the market is poised for growth. In 2025, R&D spending in the pharmaceutical sector is expected to reach approximately $5 billion, with a significant portion directed towards virtual trials. This influx of capital is likely to enhance the capabilities of clinical trial sponsors, enabling them to conduct more comprehensive and efficient trials. As a result, the virtual clinical-trials market is anticipated to expand, fostering innovation and improving the overall landscape of clinical research.