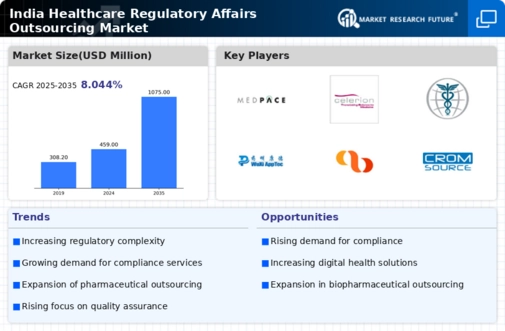
The India Healthcare Regulatory Affairs Outsourcing Market is a dynamic segment characterized by rapid evolution and a continuously shifting landscape. As pharmaceutical and biotechnology companies increasingly turn to outsourcing to streamline their processes, enhance efficiency, and navigate the complexities of regulatory compliance, the market is witnessing robust growth. Regulatory affairs outsourcing encompasses a range of services, including regulatory submissions, clinical trial management, pharmacovigilance, and market access support. This increased demand for regulatory compliance has attracted various players to enter the market, resulting in heightened competition.
Companies in this space are focusing on building expertise, expanding their service offerings, and leveraging technology to remain agile and responsive to client needs. The evolving regulatory environment in India, marked by the growing emphasis on standardization and safety, further accentuates the importance of specialized regulatory affairs outsourcing services.
Covance operates effectively within the India Healthcare Regulatory Affairs Outsourcing Market, leveraging its extensive experience and global presence to offer comprehensive regulatory solutions tailored to local needs. The company's strengths lie in its ability to provide end-to-end services encompassing regulatory strategy, submission management, and clinical trial oversight. Covance's strategic approach allows it to maintain strong relationships with regulatory authorities, thereby enhancing its credibility and efficiency in the submission process. Additionally, Covance's commitment to leveraging advanced technologies and data analytics aids in streamlining regulatory workflows, optimizing timelines for clients, and ensuring compliance with the latest regulations.
By establishing a robust operational framework in India, Covance has positioned itself as a key player capable of responding to the specific regulatory needs of the region while maintaining global standards of excellence.
PAREXEL International Corporation holds a significant presence in the India Healthcare Regulatory Affairs Outsourcing Market, offering a wide range of services including regulatory consulting, clinical trial management, and market access strategies. The company has developed strong capabilities through its extensive expertise in regulatory affairs, making it a reliable partner for firms navigating India’s complex regulatory environment. PAREXEL’s strength lies in its ability to deliver tailored solutions that meet the local compliance requirements while also adhering to international standards. With a focus on innovation, the company has integrated advanced technology solutions to enhance efficiency and accuracy in regulatory submissions.
PAREXEL is well-regarded for its strategic partnerships and collaborations that bolster its service offerings within India, and its ongoing investments in training and development of local talent further enhance its competitive stance. Through its strategic initiatives, PAREXEL continues to solidify its leadership role in the India Healthcare Regulatory Affairs Outsourcing Market while also exploring potential mergers and acquisitions to expand its footprint and reinforce its market position.