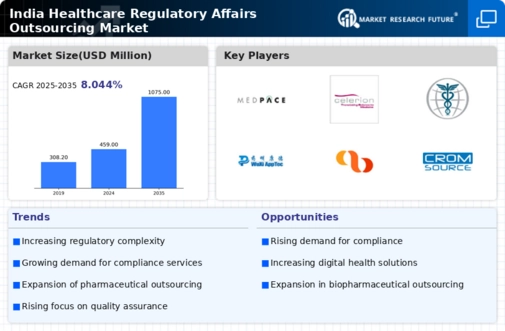
Rising Complexity of Regulations
The increasing complexity of healthcare regulations in India is a primary driver for the healthcare regulatory-affairs-outsourcing market. As the regulatory landscape evolves, companies face challenges in compliance, necessitating specialized expertise. The Indian healthcare sector has witnessed a surge in regulatory requirements, particularly with the introduction of new laws and amendments. This complexity compels organizations to seek outsourcing solutions to navigate the intricate regulatory framework effectively. It is estimated that the demand for regulatory affairs services could grow by approximately 15% annually as companies strive to maintain compliance and avoid penalties. Consequently, the healthcare regulatory-affairs-outsourcing market is likely to expand as firms prioritize regulatory compliance to ensure operational continuity.
Cost-Effectiveness of Outsourcing
The cost-effectiveness of outsourcing regulatory affairs is a significant driver for the healthcare regulatory-affairs-outsourcing market. In India, organizations are increasingly recognizing the financial benefits of outsourcing compliance functions rather than maintaining in-house teams. By outsourcing, companies can reduce operational costs and allocate resources more efficiently. It is estimated that outsourcing regulatory affairs can lead to cost savings of up to 25%, making it an attractive option for many healthcare organizations. This trend is likely to propel the growth of the healthcare regulatory-affairs-outsourcing market as firms seek to optimize their operations while ensuring compliance with regulatory requirements. As a result, the market is expected to expand as more organizations turn to outsourcing for regulatory affairs.
Increased Focus on Patient Safety
The heightened emphasis on patient safety is a crucial driver for the healthcare regulatory-affairs-outsourcing market. In India, regulatory bodies are intensifying their scrutiny of healthcare practices to ensure patient safety and quality of care. This focus has led to stricter compliance requirements, compelling healthcare organizations to seek external expertise in regulatory affairs. The market for regulatory outsourcing is projected to grow as companies prioritize patient safety and strive to meet regulatory standards. It is estimated that organizations investing in regulatory compliance can reduce adverse events by up to 30%, underscoring the importance of outsourcing regulatory affairs. Consequently, the healthcare regulatory-affairs-outsourcing market is likely to expand as firms align their operations with patient safety initiatives.
Globalization of Healthcare Services
The globalization of healthcare services is driving the healthcare regulatory-affairs-outsourcing market in India. As Indian healthcare organizations expand their reach internationally, they encounter diverse regulatory environments that require specialized knowledge. This globalization trend necessitates outsourcing regulatory affairs to ensure compliance with various international standards and regulations. The healthcare sector in India is increasingly participating in global markets, which is expected to boost the demand for regulatory expertise. It is projected that the outsourcing market could grow by 10% annually as companies seek to navigate the complexities of international regulations. Thus, the healthcare regulatory-affairs-outsourcing market is likely to benefit from the globalization of healthcare services.
Technological Advancements in Compliance
Technological advancements are significantly influencing the healthcare regulatory-affairs-outsourcing market. The integration of digital tools and platforms enhances the efficiency of compliance processes, allowing organizations to manage regulatory requirements more effectively. In India, the adoption of artificial intelligence and machine learning in regulatory affairs is on the rise, streamlining data management and analysis. This trend is expected to drive market growth, as companies increasingly rely on technology to ensure compliance with evolving regulations. Reports suggest that the use of technology in regulatory affairs could reduce compliance costs by up to 20%, making outsourcing an attractive option for many organizations. As a result, the healthcare regulatory-affairs-outsourcing market is poised for growth as firms leverage technology to enhance their compliance capabilities.
















Leave a Comment