Clinical Trials Market Trends
Clinical Trials Market Research Report By Phase (Phase I, Phase II, Phase III, Phase IV), By Study Design (Interventional, Observational, Expanded Access), By Therapeutic Area (Oncology, Cardiology, Neurology, Infectious Diseases, Endocrinology), By End Use (Pharmaceutical Companies, Biotechnology Companies, Contract Research Organizations) and By Regional (North America, Europe, South America,...
Market Summary
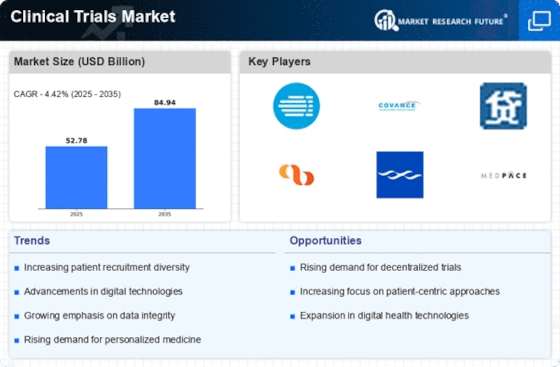
As per Market Research Future Analysis, the Clinical Trials Market was valued at 50.55 USD Billion in 2023 and is projected to grow to 85 USD Billion by 2035, with a CAGR of 4.43% from 2025 to 2035. The market is driven by the increasing demand for innovative therapies, rising chronic disease prevalence, and advancements in technology.
Key Market Trends & Highlights
The Clinical Trials Market is witnessing transformative trends that enhance its growth potential.
- The market is expected to reach 52.79 USD Billion in 2024.
- Phase III trials dominate with a projected value of 30.0 USD Billion by 2035.
- North America leads the market with a valuation of 20.0 USD Billion in 2024, growing to 32.0 USD Billion by 2035.
- The biopharmaceutical sector is growing at a CAGR of 10%, driving increased clinical trial activity.
Market Size & Forecast
| 2023 Market Size | USD 50.55 Billion |
| 2024 Market Size | USD 52.79 Billion |
| 2035 Market Size | USD 85.0 Billion |
| CAGR (2025-2035) | 4.43% |
| Largest Regional Market Share in 2024 | North America. |
Major Players
Key players include Pfizer, Merck, Charles River Laboratories, Medpace, AbbVie, Syneos Health, Bristol Myers Squibb, Johnson and Johnson, Eli Lilly and Company, PAREXEL International, Celerion, Covance, AstraZeneca, Quintiles IMS, and Icon plc.
Market Trends
The Clinical Trials Market is experiencing several important trends driven primarily by the need for more efficient and streamlined research processes. One key market driver is the increasing prevalence of chronic diseases and the rising demand for personalized medicine. This drives pharmaceutical companies and research organizations to invest heavily in clinical trials to develop innovative therapeutic solutions tailored to individual patient needs.
Moreover, regulatory authorities across various countries have begun to adopt faster approval processes for new drugs and therapies, encouraging the initiation of clinical trials. There are several opportunities to be explored in the Clinical Trials Market, particularly in the realm of digital technology.
The integration of artificial intelligence and Machine Learning in clinical trial design and management presents a significant avenue for efficiency and precision. Moreover, the use of mobile health technologies and telemedicine can facilitate patient recruitment and engagement, which is essential for the success of clinical trials, especially in remote areas.
Trends in recent times include a growing focus on decentralized clinical trials, which allow for a more patient-centric approach. This trend is increasingly relevant in today’s global landscape where remote monitoring and data collection are becoming the norm. Additionally, there is a greater emphasis on diversity and inclusion in clinical trials, as trial sponsors recognize the importance of studying diverse populations to ensure that new therapies are safe and effective for all patient demographics.
These combined trends signify a shift towards more adaptive and inclusive clinical trial practices, catering to the evolving needs of global health care.
The Global Clinical Trials Market is poised for robust growth, driven by increasing investments in research and development, alongside a rising demand for innovative therapies and personalized medicine.
U.S. Food and Drug Administration (FDA)
Clinical Trials Market Market Drivers
Market Growth Projections
The Global Clinical Trials Market Industry is on a trajectory of growth, with projections indicating a market size of 52.8 USD Billion in 2024 and an anticipated increase to 85 USD Billion by 2035. This growth reflects a compound annual growth rate of 4.43% from 2025 to 2035, driven by factors such as technological advancements, increased investment in R&D, and rising health awareness. The expansion of clinical trials across various therapeutic areas is likely to contribute to this upward trend, as stakeholders recognize the importance of clinical research in developing new therapies and improving patient outcomes.
Growing Global Health Awareness
The Global Clinical Trials Market Industry is benefiting from a heightened awareness of health issues among the global population. As individuals become more informed about diseases and treatment options, there is an increased willingness to participate in clinical trials. This trend is particularly pronounced in emerging markets, where health education campaigns are gaining traction. The growing demand for effective treatments is prompting sponsors to conduct more trials, thereby expanding the market. The anticipated growth to 85 USD Billion by 2035 underscores the importance of public engagement in clinical research and its role in advancing healthcare solutions.
Rising Demand for Innovative Therapies
The Global Clinical Trials Market Industry is experiencing a surge in demand for innovative therapies, particularly in oncology and rare diseases. As pharmaceutical companies strive to develop groundbreaking treatments, the number of clinical trials is increasing. In 2024, the market is projected to reach 52.8 USD Billion, driven by advancements in personalized medicine and biotechnology. Regulatory bodies are also facilitating faster approvals, which encourages more companies to invest in clinical trials. This trend suggests that the industry will continue to expand, with a projected market size of 85 USD Billion by 2035, reflecting a growing commitment to addressing unmet medical needs.
Regulatory Support and Streamlined Processes
Regulatory support plays a pivotal role in the Global Clinical Trials Market Industry, as agencies work to streamline processes and reduce barriers to entry. Initiatives aimed at expediting the approval of clinical trials are encouraging more companies to engage in research. The establishment of clear guidelines and frameworks fosters a conducive environment for innovation. As a result, the industry is poised for growth, with a projected market value of 52.8 USD Billion in 2024. This regulatory landscape not only enhances the efficiency of trial execution but also instills confidence in sponsors, further driving participation in clinical research.
Technological Advancements in Trial Management
Technological innovations are reshaping the Global Clinical Trials Market Industry, enhancing efficiency and data accuracy. The adoption of electronic data capture, remote monitoring, and artificial intelligence is streamlining trial processes and improving patient recruitment. These advancements not only reduce costs but also accelerate timelines, making clinical trials more attractive to sponsors. As a result, the industry is likely to see a compound annual growth rate of 4.43% from 2025 to 2035. The integration of technology into trial management systems is expected to facilitate better patient engagement and data collection, ultimately leading to more successful outcomes.
Increasing Investment in Research and Development
Investment in research and development is a critical driver of the Global Clinical Trials Market Industry. Pharmaceutical and biotechnology companies are allocating substantial resources to R&D, aiming to discover new drugs and therapies. This trend is evident as companies recognize the potential for high returns on investment in clinical trials. The market's growth is further supported by government initiatives that promote funding for clinical research. As the industry evolves, the influx of capital is likely to enhance the quality and quantity of clinical trials, contributing to the projected market size of 85 USD Billion by 2035.
Market Segment Insights
Clinical Trials Market Phase Insights
The Clinical Trials Market is characterized by a distinct segmentation into various phases, each playing a crucial role in the research and development of new therapeutics and interventions. In 2024, the Phase I segment is valued at 9.86 USD Billion, representing a foundational step in clinical research that focuses on assessing the safety, dosage, and side effects of new drugs in a small group of healthy volunteers.
This phase lays the groundwork for further testing by ensuring that the treatments are not only safe but also effective.
The Phase II segment, valued at 12.99 USD Billion in the same year, is significant as it explores the efficacy of these treatments in a larger patient population, allowing researchers to identify optimal dosages and further evaluate the treatment's safety profile.Phase III, dominating the landscape with a valuation of 22.91 USD Billion in 2024, is pivotal as it involves expansive trials that confirm the effectiveness of new therapies and compare them against existing standards of care, ultimately providing vital data for regulatory approval.
The importance of this phase cannot be overstated, as it accounts for the majority holding within the Clinical Trials Market, directly influencing market growth and advancements in medical practice. Lastly, the Phase IV segment, valued at 7.03 USD Billion, takes place after a treatment has been approved and is on the market, focusing on long-term effectiveness and uncovering any rare side effects that may emerge over extended use.Together, these phases represent a comprehensive approach to clinical research, highlighting how each stage contributes to the successful development of new medical therapies and the overall growth of the Clinical Trials Market industry.
The ongoing advancements in technology and methodologies applied during these phases are expected to drive efficiencies and enhance data integrity in clinical studies, further propelling the market forward while meeting the growing demand for innovative healthcare solutions.
Clinical Trials Market Study Design Insights
The Clinical Trials Market has witnessed a notable focus on the Study Design segment, which plays a crucial role in shaping the direction and outcomes of clinical research. The market is expected to be valued at 52.79 USD Billion in 2024, demonstrating its increasing significance within the broader clinical trial landscape.
Various methodologies exist within this segment, including interventional studies, observational studies, and expanded access protocols. Interventional studies are vital as they directly test new treatments, often leading to innovative therapies that can revolutionize patient care.Observational studies, on the other hand, provide essential insights into real-world effectiveness and safety, contributing significantly to post-marketing surveillance. Expanded access offers patients with critical conditions early access to investigational therapies, highlighting ethical elements in research.
This combination of methodologies not only enriches the Clinical Trials Market data but also supports the growing need for robust evidence in healthcare decision-making. As the demand for more personalized medicine rises, the importance of varied study designs becomes ever more pronounced, reflecting the market's responsiveness to evolving medical challenges.Overall, market growth in this domain represents the commitment to advancing healthcare through meticulous research methodologies, ensuring a well-rounded exploration of treatment avenues.
Clinical Trials Market Therapeutic Area Insights
The Clinical Trials Market is projected to reach a valuation of 52.79 billion USD by 2024, reflecting the growing importance of Clinical Trials in various Therapeutic Areas. The analysis of Clinical Trials Market data reveals significant trends driven by the increasing incidence of chronic diseases and the demand for innovative therapies.
Within Therapeutic Areas, Oncology has become increasingly vital due to the rising cancer rates worldwide, necessitating ongoing research and clinical trials in this domain. Cardiology is another essential area where clinical trials play a critical role in evaluating new treatments and interventions for heart diseases, which are among the leading causes of mortality globally.Neurology also holds a prominent place within the market, with an emphasis on addressing neurodegenerative conditions through targeted clinical studies. Furthermore, Infectious Diseases treatment have gained unprecedented attention amidst global health crises, increasing the demand for clinical trials to develop vaccines and antiviral therapies.
Endocrinology continues to be a significant area due to growing concerns regarding diabetes and hormonal disorders, necessitating robust clinical research. Overall, the Clinical Trials Market segmentation across these Therapeutic Areas underscores the need for ongoing innovation and effective solutions in the healthcare landscape, aligning with the rising global healthcare expenditures.
Clinical Trials Market End Use Insights
The Clinical Trials Market is projected to witness significant expansion, with an expected valuation of 52.79 USD Billion by 2024 and reaching approximately 85.0 USD Billion by 2035, reflecting a steady growth trajectory. Within the End Use segment, Pharmaceutical Companies play a crucial role in conducting clinical trials as they are driven by the need for drug development and regulatory approvals.
These organizations dominate the market, accounting for a substantial share due to their vast resources and expertise in Research and Development processes.Biotechnology Companies also represent a vital portion of this market, focusing on innovative therapies that necessitate robust clinical trial frameworks to ensure safety and effectiveness. Contract Research Organizations provide essential support to both pharmaceutical and biotechnology entities by managing trial logistics, thereby streamlining processes and reducing costs.
This collaborative landscape enhances efficiency in clinical trial execution, contributing to the overall growth of the Clinical Trials Market. Key drivers of this market include increasing investments in healthcare innovation, rising prevalence of chronic diseases, and a growing emphasis on personalized medicine.However, challenges such as regulatory compliance, patient recruitment, and data management remain critical considerations for stakeholders in this domain.
Get more detailed insights about Clinical Trials Market Research Report- Forecast till 2035
Regional Insights
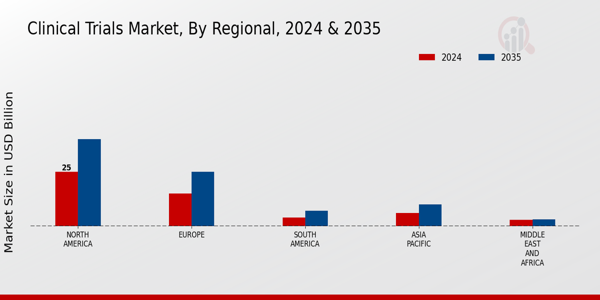
The Regional segment of the Clinical Trials Market reveals significant variations in market valuation and growth potential across different areas. In 2024, North America leads this segment with a valuation of 25.0 USD Billion, expected to reach 40.0 USD Billion by 2035, thus dominating the market due to advanced healthcare infrastructure and substantial investments in Research and Development.
Europe follows with a valuation of 15.0 USD Billion in 2024, increasing to 25.0 USD Billion in 2035, driven by robust regulatory frameworks that support clinical research.South America, while smaller, shows promise with a growth from 4.0 USD Billion in 2024 to 7.0 USD Billion in 2035, as increasing collaboration with global pharmaceutical companies enhances capabilities. Asia Pacific, valued at 6.0 USD Billion in 2024 and projected to grow to 10.0 USD Billion by 2035, benefits from a large patient population and rising medical tourism, making it an attractive landscape for clinical trials.
Lastly, the Middle East and Africa, though valued at 2.79 USD Billion in 2024, are witnessing a shift with investments aimed at improving healthcare and research capabilities, spotlighting them as a growing contributor within the Clinical Trials Market.
Each region's distinct market drivers reflect broader trends in healthcare innovation, regulatory environments, and economic conditions, underscoring varied opportunities and challenges in the clinical trials landscape.
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
Key Players and Competitive Insights
The Clinical Trials Market is characterized by intense competition fueled by the rapid advancements in biotechnology, pharmaceuticals, and medical devices. Companies engaged in this sector are focusing on enhancing their capabilities to conduct comprehensive trials, which is essential for meeting regulatory requirements and overcoming market challenges. The competitive landscape is shaped by factors such as the increasing prevalence of chronic diseases, a surge in the need for novel therapies, and a heightened emphasis on personalized medicine.
The market is also witnessing significant investments in technology, including artificial intelligence and data analytics, which are integral to optimizing trial processes, reducing timeframes, and improving patient recruitment rates. The global nature of the clinical trials market also allows firms to broaden their geographical reach and tap into emerging economies, which is influencing competitive strategies.Parexel International has established itself as a leading player in the Clinical Trials Market, renowned for its extensive experience and expertise in providing comprehensive drug development solutions.
The company has built a strong reputation through its capability to assist clients across various phases of clinical trials, thereby strengthening its market presence. With an extensive global operational network, Parexel International has the advantage of access to diverse patient populations, allowing it to facilitate quicker recruitment.
This operational efficiency, combined with its robust regulatory knowledge and an emphasis on quality, positions the company as a preferred partner for many biotech and pharmaceutical firms. The company's ability to offer strategic consulting services and innovative solutions further enhances its appeal in a complex market landscape.Medpace is another significant player in the Clinical Trials Market, known for its specialized expertise in managing multidisciplinary clinical trials across various therapeutic areas. The company offers a range of key services, including study design, project management, and regulatory support, which are tailored to meet the specific needs of its clients.
Medpace's strong commitment to quality and adherence to regulatory standards has solidified its position in the market.
The company has expanded its global presence through strategic mergers and acquisitions, enhancing its capacity to provide integrated solutions for clinical development. With a focus on fostering long-term relationships with clients, Medpace's strengths lie in its extensive experience, a strong scientific foundation, and a dedicated operational team that drives project success. These factors collectively enhance its competitiveness within the Clinical Trials Market.
Key Companies in the Clinical Trials Market market include








Industry Developments
Recent developments in the Clinical Trials Market include several significant advancements and ongoing trends resulting from increasing investments in healthcare innovation. In July 2023, Parexel International announced an expansion of its clinical trial services to include advanced data analytics, enhancing study efficiency. Medpace has recently seen a marked increase in demand for its clinical development services as pharmaceutical companies seek faster trial timelines.
Charles River Laboratories and PRA Health Sciences are exploring partnerships focused on accelerating drug development processes through digital health technologies, reflecting a broader industry trend towards integrating technology into clinical trials.
Mergers and acquisitions have also shaped the market landscape, with IQVIA acquiring a minor stake in Synlogic in August 2022, allowing for a stronger emphasis on precision medicine. Additionally, Wuxi AppTec has branched out with new collaborative efforts to optimize global clinical trial strategies.
The market has witnessed substantial growth, bolstered by the rising necessity for innovative treatments and a streamlined regulatory environment, indicating a robust and evolving clinical landscape as stakeholders adapt to changes in patient needs and advanced trial methodologies. Major activities over the past few years have emphasized data-driven approaches, enhancing patient engagement, and utilizing decentralized trial models for improved efficiency.
Future Outlook
Clinical Trials Market Future Outlook
The Global Clinical Trials Market is projected to grow at a 4.43% CAGR from 2024 to 2035, driven by technological advancements, increasing R&D investments, and a rising demand for personalized medicine.
New opportunities lie in:
- Leverage AI-driven analytics to enhance patient recruitment and retention strategies.
- Develop partnerships with biotech firms to streamline trial processes and reduce costs.
- Invest in decentralized trial models to improve accessibility and patient engagement.
By 2035, the Global Clinical Trials Market is expected to be robust, reflecting substantial growth and innovation.
Market Segmentation
Clinical Trials Market Phase Outlook
- Phase I
- Phase II
- Phase III
- Phase IV
Clinical Trials Market End Use Outlook
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
Clinical Trials Market Regional Outlook
- North America
- Europe
- South America
- Asia Pacific
- Middle East and Africa
Clinical Trials Market Study Design Outlook
- Interventional
- Observational
- Expanded Access
Clinical Trials Market Therapeutic Area Outlook
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
Report Scope
|
Report Attribute/Metric |
Details |
|
Market Size 2023 |
50.55(USD Billion) |
|
Market Size 2024 |
52.79(USD Billion) |
|
Market Size 2035 |
85.0(USD Billion) |
|
Compound Annual Growth Rate (CAGR) |
4.42% (2025 - 2035) |
|
Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
|
Base Year |
2024 |
|
Market Forecast Period |
2025 - 2035 |
|
Historical Data |
2019 - 2024 |
|
Market Forecast Units |
USD Billion |
|
Key Companies Profiled |
Parexel International, Medpace, Charles River Laboratories, Clinipace, PPD, PRA Health Sciences, IQVIA, EPS International, Syneos Health, Synlogic, KCR, Wuxi AppTec, Pharmaceutical Product Development, BioClinica, Covance |
|
Segments Covered |
Phase, Study Design, Therapeutic Area, End Use, Regional |
|
Key Market Opportunities |
Increased demand for personalized medicine, Growth of decentralized clinical trials, Advancements in data analytics tools, Expansion in emerging markets, Rise in collaborations between biotech firms. |
|
Key Market Dynamics |
Rising demand for novel therapies, Increasing patient recruitment challenges, Regulatory compliance complexities, Growing adoption of technology solutions, and Expansion of personalized medicine approaches |
|
Countries Covered |
North America, Europe, APAC, South America, MEA |
Market Highlights
Author
Latest Comments
This is a great article! Really helped me understand the topic better.
Thanks for sharing this. I’ve bookmarked it for later reference.
FAQs
What was the market size of the Clinical Trials Market in 2024?
The Clinical Trials Market was valued at 52.79 USD Billion in 2024.
What is the projected market value of the Clinical Trials Market by 2035?
By 2035, the Clinical Trials Market is projected to reach a value of 85.0 USD Billion.
What is the expected CAGR for the Clinical Trials Market from 2025 to 2035?
The expected CAGR for the Clinical Trials Market from 2025 to 2035 is 4.42%.
Which region holds the largest market share in the Clinical Trials Market for 2024?
North America holds the largest market share with a value of 25.0 USD Billion in 2024.
What will be the market value of Europe in the Clinical Trials Market by 2035?
Europe's market value in the Clinical Trials Market is anticipated to be 25.0 USD Billion by 2035.
What are the expected market values for Phase II Clinical Trials in 2024 and 2035?
Phase II Clinical Trials are valued at 12.99 USD Billion in 2024 and expected to reach 20.55 USD Billion in 2035.
Who are the key players in the Clinical Trials Market?
Key players in the Clinical Trials Market include Parexel International, Medpace, and IQVIA, among others.
What is the projected growth rate for the Asia Pacific region in the Clinical Trials Market from 2024 to 2035?
The Asia Pacific region is expected to grow from 6.0 USD Billion in 2024 to 10.0 USD Billion by 2035.
What was the market value of Phase III Clinical Trials in 2024?
Phase III Clinical Trials segment was valued at 22.91 USD Billion in 2024.
What challenges and opportunities are present in the Clinical Trials Market?
The Clinical Trials Market faces challenges such as regulatory hurdles, but also presents opportunities driven by increasing research and development investment.
-
Table of Contents
-
EXECUTIVE SUMMARY
- Market Overview
- Key Findings
- Market Segmentation
- Competitive Landscape
- Challenges and Opportunities
- Future Outlook
-
MARKET INTRODUCTION
- Definition
-
Scope of the study
- Research Objective
- Assumption
- Limitations
-
RESEARCH METHODOLOGY
- Overview
- Data Mining
- Secondary Research
-
Primary Research
- Primary Interviews and Information Gathering Process
- Breakdown of Primary Respondents
- Forecasting Model
-
Market Size Estimation
- Bottom-Up Approach
- Top-Down Approach
- Data Triangulation
- Validation
-
MARKET DYNAMICS
- Overview
- Drivers
- Restraints
- Opportunities
-
MARKET FACTOR ANALYSIS
- Value chain Analysis
-
Porter's Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
COVID-19 Impact Analysis
- Market Impact Analysis
- Regional Impact
- Opportunity and Threat Analysis
-
Clinical Trials Market, BY Phase (USD Billion)
- Phase I
- Phase II
- Phase III
- Phase IV
-
Clinical Trials Market, BY Study Design (USD Billion)
- Interventional
- Observational
- Expanded Access
-
Clinical Trials Market, BY Therapeutic Area (USD Billion)
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
-
Clinical Trials Market, BY End Use (USD Billion)
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
-
Clinical Trials Market, BY Regional (USD Billion)
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Russia
- Italy
- Spain
- Rest of Europe
-
APAC
- China
- India
- Japan
- South Korea
- Malaysia
- Thailand
- Indonesia
- Rest of APAC
-
South America
- Brazil
- Mexico
- Argentina
- Rest of South America
-
MEA
- GCC Countries
- South Africa
- Rest of MEA
-
North America
-
Competitive Landscape
- Overview
- Competitive Analysis
- Market share Analysis
- Major Growth Strategy in the Clinical Trials Market
- Competitive Benchmarking
- Leading Players in Terms of Number of Developments in the Clinical Trials Market
-
Key developments and growth strategies
- New Product Launch/Service Deployment
- Merger & Acquisitions
- Joint Ventures
-
Major Players Financial Matrix
- Sales and Operating Income
- Major Players R&D Expenditure. 2023
-
Company Profiles
-
Pfizer
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Merck
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Charles River Laboratories
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Medpace
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
AbbVie
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Syneos Health
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Bristol Myers Squibb
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Johnson and Johnson
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Eli Lilly and Company
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
PAREXEL International
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Celerion
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Covance
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
AstraZeneca
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Quintiles IMS
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Icon plc
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Pfizer
-
Appendix
- References
- Related Reports
-
List of Tables and Figures
- LIST OF ASSUMPTIONS
- North America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- North America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- North America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- North America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- North America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- US Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- US Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- US Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- US Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- US Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Canada Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Canada Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Canada Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Canada Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Canada Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Germany Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Germany Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Germany Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Germany Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Germany Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- UK Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- UK Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- UK Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- UK Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- UK Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- France Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- France Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- France Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- France Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- France Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Russia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Russia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Russia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Russia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Russia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Italy Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Italy Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Italy Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Italy Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Italy Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Spain Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Spain Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Spain Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Spain Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Spain Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Rest of Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Rest of Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Rest of Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Rest of Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Rest of Europe Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- China Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- China Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- China Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- China Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- China Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- India Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- India Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- India Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- India Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- India Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Japan Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Japan Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Japan Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Japan Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Japan Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- South Korea Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- South Korea Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- South Korea Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- South Korea Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- South Korea Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Malaysia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Malaysia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Malaysia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Malaysia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Malaysia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Thailand Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Thailand Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Thailand Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Thailand Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Thailand Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Indonesia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Indonesia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Indonesia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Indonesia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Indonesia Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Rest of APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Rest of APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Rest of APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Rest of APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Rest of APAC Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Brazil Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Brazil Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Brazil Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Brazil Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Brazil Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Mexico Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Mexico Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Mexico Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Mexico Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Mexico Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Argentina Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Argentina Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Argentina Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Argentina Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Argentina Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Rest of South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Rest of South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Rest of South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Rest of South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Rest of South America Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- GCC Countries Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- GCC Countries Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- GCC Countries Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- GCC Countries Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- GCC Countries Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- South Africa Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- South Africa Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- South Africa Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- South Africa Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- South Africa Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- Rest of MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY PHASE, 2019-2035 (USD Billions)
- Rest of MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY STUDY DESIGN, 2019-2035 (USD Billions)
- Rest of MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
- Rest of MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY END USE, 2019-2035 (USD Billions)
- Rest of MEA Clinical Trials Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
- PRODUCT LAUNCH/PRODUCT DEVELOPMENT/APPROVAL
- ACQUISITION/PARTNERSHIP
- MARKET SYNOPSIS
- NORTH AMERICA CLINICAL TRIALS MARKET ANALYSIS
- US CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- US CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- US CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- US CLINICAL TRIALS MARKET ANALYSIS BY END USE
- US CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- CANADA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- CANADA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- CANADA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- CANADA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- CANADA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- EUROPE CLINICAL TRIALS MARKET ANALYSIS
- GERMANY CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- GERMANY CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- GERMANY CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- GERMANY CLINICAL TRIALS MARKET ANALYSIS BY END USE
- GERMANY CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- UK CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- UK CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- UK CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- UK CLINICAL TRIALS MARKET ANALYSIS BY END USE
- UK CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- FRANCE CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- FRANCE CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- FRANCE CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- FRANCE CLINICAL TRIALS MARKET ANALYSIS BY END USE
- FRANCE CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- RUSSIA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- RUSSIA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- RUSSIA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- RUSSIA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- RUSSIA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- ITALY CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- ITALY CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- ITALY CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- ITALY CLINICAL TRIALS MARKET ANALYSIS BY END USE
- ITALY CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- SPAIN CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- SPAIN CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- SPAIN CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- SPAIN CLINICAL TRIALS MARKET ANALYSIS BY END USE
- SPAIN CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- REST OF EUROPE CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- REST OF EUROPE CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- REST OF EUROPE CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- REST OF EUROPE CLINICAL TRIALS MARKET ANALYSIS BY END USE
- REST OF EUROPE CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- APAC CLINICAL TRIALS MARKET ANALYSIS
- CHINA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- CHINA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- CHINA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- CHINA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- CHINA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- INDIA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- INDIA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- INDIA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- INDIA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- INDIA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- JAPAN CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- JAPAN CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- JAPAN CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- JAPAN CLINICAL TRIALS MARKET ANALYSIS BY END USE
- JAPAN CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- SOUTH KOREA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- SOUTH KOREA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- SOUTH KOREA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- SOUTH KOREA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- SOUTH KOREA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- MALAYSIA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- MALAYSIA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- MALAYSIA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- MALAYSIA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- MALAYSIA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- THAILAND CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- THAILAND CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- THAILAND CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- THAILAND CLINICAL TRIALS MARKET ANALYSIS BY END USE
- THAILAND CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- INDONESIA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- INDONESIA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- INDONESIA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- INDONESIA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- INDONESIA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- REST OF APAC CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- REST OF APAC CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- REST OF APAC CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- REST OF APAC CLINICAL TRIALS MARKET ANALYSIS BY END USE
- REST OF APAC CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- SOUTH AMERICA CLINICAL TRIALS MARKET ANALYSIS
- BRAZIL CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- BRAZIL CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- BRAZIL CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- BRAZIL CLINICAL TRIALS MARKET ANALYSIS BY END USE
- BRAZIL CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- MEXICO CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- MEXICO CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- MEXICO CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- MEXICO CLINICAL TRIALS MARKET ANALYSIS BY END USE
- MEXICO CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- ARGENTINA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- ARGENTINA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- ARGENTINA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- ARGENTINA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- ARGENTINA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- REST OF SOUTH AMERICA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- REST OF SOUTH AMERICA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- REST OF SOUTH AMERICA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- REST OF SOUTH AMERICA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- REST OF SOUTH AMERICA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- MEA CLINICAL TRIALS MARKET ANALYSIS
- GCC COUNTRIES CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- GCC COUNTRIES CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- GCC COUNTRIES CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- GCC COUNTRIES CLINICAL TRIALS MARKET ANALYSIS BY END USE
- GCC COUNTRIES CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- SOUTH AFRICA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- SOUTH AFRICA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- SOUTH AFRICA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- SOUTH AFRICA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- SOUTH AFRICA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- REST OF MEA CLINICAL TRIALS MARKET ANALYSIS BY PHASE
- REST OF MEA CLINICAL TRIALS MARKET ANALYSIS BY STUDY DESIGN
- REST OF MEA CLINICAL TRIALS MARKET ANALYSIS BY THERAPEUTIC AREA
- REST OF MEA CLINICAL TRIALS MARKET ANALYSIS BY END USE
- REST OF MEA CLINICAL TRIALS MARKET ANALYSIS BY REGIONAL
- KEY BUYING CRITERIA OF CLINICAL TRIALS MARKET
- RESEARCH PROCESS OF MRFR
- DRO ANALYSIS OF CLINICAL TRIALS MARKET
- DRIVERS IMPACT ANALYSIS: CLINICAL TRIALS MARKET
- RESTRAINTS IMPACT ANALYSIS: CLINICAL TRIALS MARKET
- SUPPLY / VALUE CHAIN: CLINICAL TRIALS MARKET
- CLINICAL TRIALS MARKET, BY PHASE, 2025 (% SHARE)
- CLINICAL TRIALS MARKET, BY PHASE, 2019 TO 2035 (USD Billions)
- CLINICAL TRIALS MARKET, BY STUDY DESIGN, 2025 (% SHARE)
- CLINICAL TRIALS MARKET, BY STUDY DESIGN, 2019 TO 2035 (USD Billions)
- CLINICAL TRIALS MARKET, BY THERAPEUTIC AREA, 2025 (% SHARE)
- CLINICAL TRIALS MARKET, BY THERAPEUTIC AREA, 2019 TO 2035 (USD Billions)
- CLINICAL TRIALS MARKET, BY END USE, 2025 (% SHARE)
- CLINICAL TRIALS MARKET, BY END USE, 2019 TO 2035 (USD Billions)
- CLINICAL TRIALS MARKET, BY REGIONAL, 2025 (% SHARE)
- CLINICAL TRIALS MARKET, BY REGIONAL, 2019 TO 2035 (USD Billions)
- BENCHMARKING OF MAJOR COMPETITORS
Clinical Trials Market Segmentation
Clinical Trials Market By Phase (USD Billion, 2019-2035)
- Phase I
- Phase II
- Phase III
- Phase IV
Clinical Trials Market By Study Design (USD Billion, 2019-2035)
- Interventional
- Observational
- Expanded Access
Clinical Trials Market By Therapeutic Area (USD Billion, 2019-2035)
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
Clinical Trials Market By End Use (USD Billion, 2019-2035)
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
Clinical Trials Market By Regional (USD Billion, 2019-2035)
- North America
- Europe
- South America
- Asia Pacific
- Middle East and Africa
Clinical Trials Market Regional Outlook (USD Billion, 2019-2035)
North America Outlook (USD Billion, 2019-2035)
North America Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
North America Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
North America Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
North America Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
North America Clinical Trials Market by Regional Type
- US
- Canada
- US Outlook (USD Billion, 2019-2035)
US Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
US Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
US Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
US Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- CANADA Outlook (USD Billion, 2019-2035)
CANADA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
CANADA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
CANADA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
CANADA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
Europe Outlook (USD Billion, 2019-2035)
Europe Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
Europe Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
Europe Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
Europe Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
Europe Clinical Trials Market by Regional Type
- Germany
- UK
- France
- Russia
- Italy
- Spain
- Rest of Europe
- GERMANY Outlook (USD Billion, 2019-2035)
GERMANY Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
GERMANY Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
GERMANY Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
GERMANY Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- UK Outlook (USD Billion, 2019-2035)
UK Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
UK Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
UK Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
UK Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- FRANCE Outlook (USD Billion, 2019-2035)
FRANCE Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
FRANCE Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
FRANCE Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
FRANCE Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- RUSSIA Outlook (USD Billion, 2019-2035)
RUSSIA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
RUSSIA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
RUSSIA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
RUSSIA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- ITALY Outlook (USD Billion, 2019-2035)
ITALY Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
ITALY Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
ITALY Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
ITALY Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- SPAIN Outlook (USD Billion, 2019-2035)
SPAIN Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
SPAIN Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
SPAIN Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
SPAIN Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- REST OF EUROPE Outlook (USD Billion, 2019-2035)
REST OF EUROPE Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
REST OF EUROPE Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
REST OF EUROPE Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
REST OF EUROPE Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
APAC Outlook (USD Billion, 2019-2035)
APAC Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
APAC Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
APAC Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
APAC Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
APAC Clinical Trials Market by Regional Type
- China
- India
- Japan
- South Korea
- Malaysia
- Thailand
- Indonesia
- Rest of APAC
- CHINA Outlook (USD Billion, 2019-2035)
CHINA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
CHINA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
CHINA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
CHINA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- INDIA Outlook (USD Billion, 2019-2035)
INDIA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
INDIA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
INDIA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
INDIA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- JAPAN Outlook (USD Billion, 2019-2035)
JAPAN Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
JAPAN Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
JAPAN Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
JAPAN Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- SOUTH KOREA Outlook (USD Billion, 2019-2035)
SOUTH KOREA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
SOUTH KOREA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
SOUTH KOREA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
SOUTH KOREA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- MALAYSIA Outlook (USD Billion, 2019-2035)
MALAYSIA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
MALAYSIA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
MALAYSIA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
MALAYSIA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- THAILAND Outlook (USD Billion, 2019-2035)
THAILAND Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
THAILAND Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
THAILAND Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
THAILAND Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- INDONESIA Outlook (USD Billion, 2019-2035)
INDONESIA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
INDONESIA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
INDONESIA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
INDONESIA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- REST OF APAC Outlook (USD Billion, 2019-2035)
REST OF APAC Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
REST OF APAC Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
REST OF APAC Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
REST OF APAC Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
South America Outlook (USD Billion, 2019-2035)
South America Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
South America Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
South America Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
South America Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
South America Clinical Trials Market by Regional Type
- Brazil
- Mexico
- Argentina
- Rest of South America
- BRAZIL Outlook (USD Billion, 2019-2035)
BRAZIL Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
BRAZIL Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
BRAZIL Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
BRAZIL Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- MEXICO Outlook (USD Billion, 2019-2035)
MEXICO Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
MEXICO Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
MEXICO Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
MEXICO Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- ARGENTINA Outlook (USD Billion, 2019-2035)
ARGENTINA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
ARGENTINA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
ARGENTINA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
ARGENTINA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- REST OF SOUTH AMERICA Outlook (USD Billion, 2019-2035)
REST OF SOUTH AMERICA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
REST OF SOUTH AMERICA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
REST OF SOUTH AMERICA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
REST OF SOUTH AMERICA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
MEA Outlook (USD Billion, 2019-2035)
MEA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
MEA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
MEA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
MEA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
MEA Clinical Trials Market by Regional Type
- GCC Countries
- South Africa
- Rest of MEA
- GCC COUNTRIES Outlook (USD Billion, 2019-2035)
GCC COUNTRIES Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
GCC COUNTRIES Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
GCC COUNTRIES Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
GCC COUNTRIES Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- SOUTH AFRICA Outlook (USD Billion, 2019-2035)
SOUTH AFRICA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
SOUTH AFRICA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
SOUTH AFRICA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
SOUTH AFRICA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- REST OF MEA Outlook (USD Billion, 2019-2035)
REST OF MEA Clinical Trials Market by Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
REST OF MEA Clinical Trials Market by Study Design Type
- Interventional
- Observational
- Expanded Access
REST OF MEA Clinical Trials Market by Therapeutic Area Type
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Endocrinology
REST OF MEA Clinical Trials Market by End Use Type
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations

Free Sample Request
Kindly complete the form below to receive a free sample of this Report
Customer Strories
“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”
Leave a Comment