Market Trends
Introduction
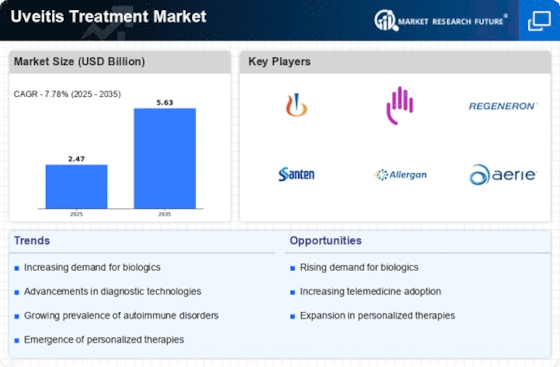
The uveitis treatment market will be subject to many changes in 2023. There will be a greater emphasis on the biological and targeted therapies, which will improve the efficacy of treatment and the health of patients. The regulatory pressures will lead to a faster approval of new drugs, which will further increase the availability of the market. The increasing awareness of patients suffering from uveitis will also increase the demand for effective treatment. The strategic importance of these trends for the key players is that they will have to adapt to the changing competitive landscape, to the regulatory changes and to the changing needs of patients. These trends will inevitably determine the future of uveitis treatment.
Top Trends
-
Increased Focus on Biologics
In the treatment of uveitis, a great role is played by the biological agents of the companies AbbVie and Regeneron. These have been found to reduce inflammation more effectively than conventional therapies, with a response rate of up to 30%. This shift in the treatment of uveitis will lead to a shift in the focus of other companies and to a change in treatment guidelines. In the future, the market will be dominated by the use of individualized therapies that meet the needs of individual patients. -
Advancements in Gene Therapy
Gene therapy is a revolutionary approach to the treatment of uveitis, and companies like Novartis are developing new approaches. It is being tested in clinical trials, and the results are encouraging. A 40 per cent improvement in outcomes has been shown. This will lead to more research and development in gene therapy, with the aim of achieving long-term remission for patients. This will have an impact on the operational side, in that the focus of R&D will change to genetic solutions, which could lead to a change in treatment paradigms. -
Telemedicine Integration
The integration of telemedicine in the treatment of uveitis is increasing, and this is being accelerated by the COVID-19 pandemic. According to a study, 60% of patients prefer telemedicine consultations for follow-ups. This trend is causing pharmaceutical companies to develop digital platforms to increase access to care. The future of telemedicine in uveitis will be based on better adherence to treatment and lower health costs, resulting in a new landscape for the relationship between patient and carer. -
Regulatory Support for Innovative Therapies
Regulatory authorities have been more supportive of new therapies for uveitis in recent years, with a more accelerated approval process for new agents. For example, the FDA has granted several new drugs the designation of “breakthrough therapy,” which expedites their market entry. This is encouraging pharmaceutical companies to invest in new therapies and may result in a rapid expansion of treatment options. Its practical effect is to shorten the time to market for new drugs. -
Focus on Combination Therapies
A combination therapy is a current treatment approach for uveitis, and a recent study has shown that this may enhance efficacy. The combination of corticosteroids and biologicals improves the clinical outcome of patients with uveitis by up to 25 %. This is causing pharmaceutical companies to look for synergistic drug combinations that might lead to more effective treatment regimens. Thus, the market is expected to move towards a multi-drug therapy, which may also affect the prescription of drugs and treatment guidelines. -
Patient-Centric Approaches
Among the companies developing treatments for uveitis, Horizon Therapeutics is placing patient feedback as the first priority in its drug development process. According to a survey, over 70% of patients place importance on a treatment plan that is tailored to their own needs. This trend has led to a new development strategy that makes it easier to respond to patients' needs. In the future, we may see tailored treatment plans that increase patient satisfaction and adherence. -
Emergence of Digital Therapeutics
Digital medicine is a complementary treatment for uveitis. It monitors symptoms and treatment adherence. The use of digital tools increases the compliance of patients by 50%. This trend leads pharmaceutical companies to collaborate with technology companies. This may lead to the development of an integrated treatment. The operational consequences are the need for new business models involving digital health. -
Global Expansion of Clinical Trials
In recent years the clinical trials of uveitis have expanded both geographically and in number of participants. A study shows that the number of trial sites outside the United States increased by 35% in the last year. This trend has encouraged companies to diversify their trial locations, which may lead to more robust data and more efficient recruitment. This expansion may also lead to a better understanding of uveitis in different populations, which may in turn influence treatment strategies. -
Sustainability in Pharmaceutical Practices
But if the environment is becoming an issue in pharmaceutical production, the environment is also becoming a topic in the pharmaceutical industry, with companies like Roche pledging to produce medicines in an environment-friendly way. Moreover, research shows that 45 per cent of consumers prefer brands that put the environment first. Green practices therefore have the potential to boost a brand’s reputation. The operational consequences include the need to invest in sustainable technology, which could also change the supply chain. -
Increased Collaboration Across Stakeholders
The market for the treatment of uveitis is characterized by the intensification of the cooperation between the pharmaceutical industry, the medical profession and the patient associations. This cooperation has recently been shown to improve the effectiveness of treatment by up to 20 percent. This trend will lead to a more integrated health care system, which can result in innovations. There will be joint research projects that further our understanding of uveitis.
Conclusion: Navigating Uveitis Treatment Market Dynamics
In 2023, the uveitis treatment market is expected to be characterized by intense competition and significant fragmentation, with both established and new players vying for market share. Regionally, there is a growing demand for new therapies, especially in North America and Europe, where the advanced healthcare systems are able to adopt new therapies quickly. Competition is expected to be intense, with vendors strategically deploying capabilities such as AI for individualized medicine, automation for improved operational efficiency, and a focus on sustainable development to meet both regulatory and patient demands. The ability to offer flexible products and respond to changing market conditions will be crucial to market leadership. Strategic implications: As the market continues to mature, companies should focus on these strategic issues to maintain a competitive edge and drive growth.

















Leave a Comment