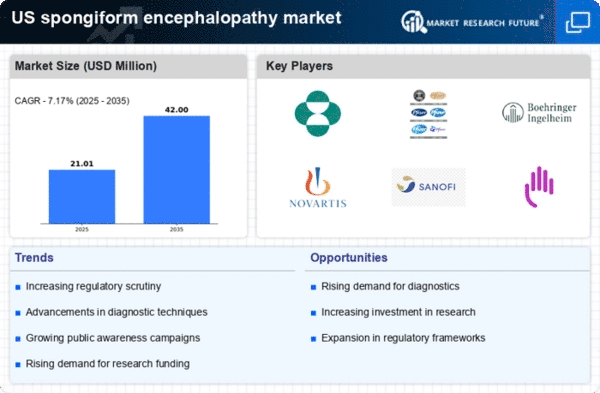
Rising Awareness of Prion Diseases
The increasing awareness of prion diseases, including spongiform encephalopathy, is a notable driver in the spongiform encephalopathy market. Educational campaigns and public health initiatives have heightened understanding of the risks associated with these diseases. As a result, healthcare professionals and the general public are more vigilant about symptoms and transmission. This awareness is likely to lead to earlier diagnosis and treatment, which could enhance market growth. Furthermore, the Centers for Disease Control and Prevention (CDC) has reported a rise in inquiries related to prion diseases, indicating a growing concern. The heightened awareness may also drive demand for diagnostic tools and therapeutic options, thereby expanding the spongiform encephalopathy market. The market could potentially see a growth rate of 5-7% annually as awareness continues to spread.
Advancements in Diagnostic Techniques
Innovations in diagnostic techniques are significantly influencing the spongiform encephalopathy market. The development of more sensitive and specific tests for detecting prion proteins has improved the accuracy of diagnoses. Techniques such as real-time quaking-induced conversion (RT-QuIC) and advanced imaging methods are becoming more prevalent. These advancements not only facilitate earlier detection but also enhance the understanding of disease progression. As diagnostic capabilities improve, healthcare providers are likely to adopt these technologies, leading to increased testing and monitoring of at-risk populations. The market for diagnostic tools in the spongiform encephalopathy market is projected to grow, with estimates suggesting a valuation of over $200 million by 2027. This growth is indicative of the critical role that accurate diagnostics play in managing prion diseases.
Growing Demand for Veterinary Diagnostics
The growing demand for veterinary diagnostics is emerging as a significant driver in the spongiform encephalopathy market. With the recognition of transmissible spongiform encephalopathies in animals, particularly in cattle and sheep, there is an increasing need for effective diagnostic tools in veterinary medicine. The spongiform encephalopathy market is witnessing a surge in demand for tests that can identify prion diseases in livestock, which is crucial for food safety and public health. The American Veterinary Medical Association (AVMA) has noted a rise in testing protocols for prion diseases, reflecting the industry's commitment to preventing outbreaks. This trend is likely to contribute to market growth, with estimates suggesting a potential increase in veterinary diagnostics revenue by 10% annually.
Increased Focus on Food Safety Regulations
The increased focus on food safety regulations is a pivotal driver in the spongiform encephalopathy market. Regulatory bodies, such as the Food and Drug Administration (FDA), are implementing stricter guidelines to prevent the transmission of prion diseases through the food supply. These regulations are aimed at ensuring that meat and animal products are free from contamination. As a result, there is a heightened demand for testing and monitoring solutions within the spongiform encephalopathy market. The emphasis on food safety is likely to drive investments in diagnostic technologies and surveillance systems. The market could potentially experience growth, with projections indicating a rise in compliance-related expenditures by 5-10% over the next few years.
Regulatory Support for Research Initiatives
Regulatory support for research initiatives is a crucial driver in the spongiform encephalopathy market. Government agencies, including the National Institutes of Health (NIH), have allocated substantial funding for research into prion diseases. This funding is aimed at understanding the mechanisms of disease transmission and developing effective treatments. The NIH has reported an increase in grants specifically targeting prion research, which could lead to breakthroughs in therapeutic options. As research progresses, new findings may emerge, potentially transforming the landscape of the spongiform encephalopathy market. The anticipated outcomes of these research initiatives could result in a market expansion, with projections indicating a compound annual growth rate (CAGR) of 6-8% over the next five years.