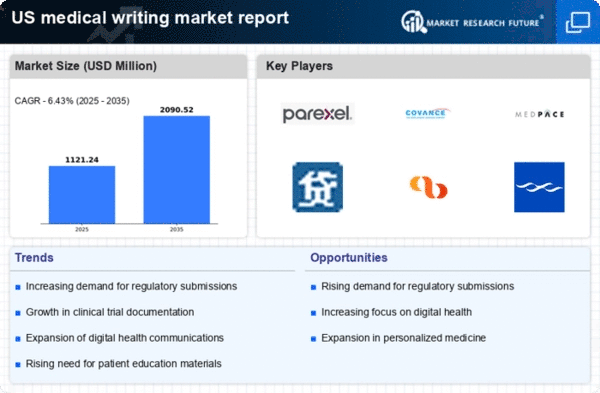
Rising Demand for Clinical Trials
The medical writing market experiences a notable surge in demand due to the increasing number of clinical trials conducted in the United States. As pharmaceutical companies and research organizations strive to bring new therapies to market, the need for precise and compliant documentation becomes paramount. In 2025, the number of clinical trials registered in the US is projected to exceed 40,000, reflecting a growth rate of approximately 10% annually. This trend necessitates skilled medical writers who can produce high-quality clinical study reports, protocols, and informed consent documents. The medical writing market is thus positioned to benefit from this expanding landscape, as the complexity of trial designs and regulatory requirements continues to evolve.
Expansion of Biopharmaceutical Sector
The biopharmaceutical sector in the United States is undergoing rapid expansion, which significantly impacts the medical writing market. With an increasing number of biopharmaceutical companies emerging, there is a heightened demand for specialized medical writing services. In 2025, the biopharmaceutical market is expected to reach a valuation of $500 billion, with a substantial portion allocated to research and development activities. This growth translates into a greater need for medical writers who can effectively communicate complex scientific data and regulatory submissions. The medical writing market is likely to see a corresponding increase in opportunities as these companies seek to navigate the intricate landscape of drug development and regulatory compliance.
Technological Advancements in Documentation
Technological advancements are reshaping the landscape of the medical writing market, introducing new tools and platforms that enhance efficiency and accuracy. The integration of artificial intelligence and machine learning in document creation and review processes is becoming more prevalent. In 2025, it is anticipated that 30% of medical writing tasks will be automated, allowing writers to focus on higher-level strategic tasks. This shift not only streamlines workflows but also improves the quality of documentation. The medical writing market is likely to benefit from these innovations, as they enable faster turnaround times and reduce the risk of errors in regulatory submissions.
Growing Importance of Regulatory Submissions
The medical writing market is increasingly influenced by the growing importance of regulatory submissions in the drug development process. As regulatory agencies demand more comprehensive and transparent documentation, the role of medical writers becomes critical. In 2025, it is projected that the number of regulatory submissions will increase by 15%, necessitating a skilled workforce capable of producing high-quality submission documents. This trend underscores the need for medical writers to stay abreast of evolving regulatory guidelines and standards. The medical writing market is thus positioned to thrive as organizations seek to ensure compliance and facilitate successful interactions with regulatory authorities.
Increased Focus on Patient-Centric Approaches
The medical writing market is witnessing a shift towards patient-centric approaches in clinical research and documentation. As stakeholders prioritize patient engagement and experience, there is a growing need for medical writers to create materials that resonate with patients. This trend is reflected in the increasing emphasis on plain language summaries and patient information leaflets. In 2025, it is estimated that 60% of clinical trial protocols will incorporate patient feedback, highlighting the importance of effective communication. The medical writing market must adapt to these evolving expectations, ensuring that documentation not only meets regulatory standards but also addresses the needs and understanding of patients.