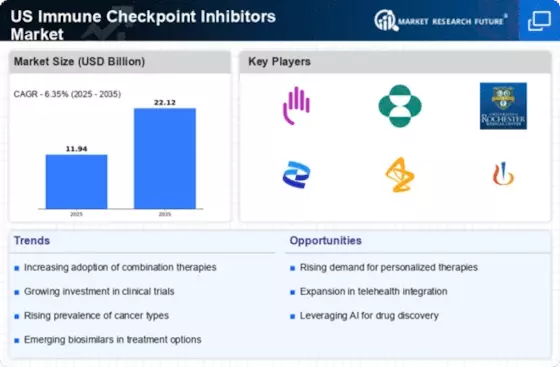
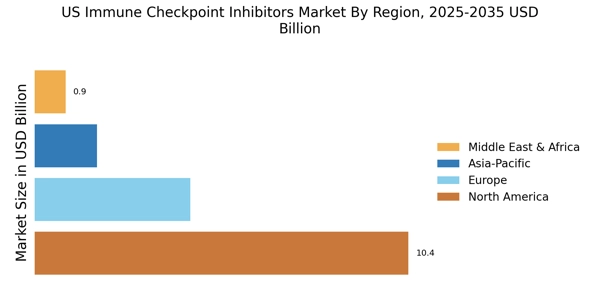
Growing Incidence of Cancer
The US Immune Checkpoint Inhibitors Market is experiencing growth due to the rising incidence of various cancers, including melanoma, lung cancer, and bladder cancer. According to the American Cancer Society, an estimated 1.9 million new cancer cases are expected to be diagnosed in the United States in 2026. This alarming statistic underscores the urgent need for effective treatment options, propelling the demand for immune checkpoint inhibitors. These therapies have shown promising results in improving patient outcomes, leading to increased adoption by healthcare providers. As more patients seek innovative treatment modalities, the market for immune checkpoint inhibitors is likely to expand, driven by the need to address the growing cancer burden in the US.
Regulatory Support and Approvals
The US Immune Checkpoint Inhibitors Market is bolstered by favorable regulatory support and expedited approval processes. The Food and Drug Administration (FDA) has implemented initiatives such as the Breakthrough Therapy Designation, which aims to expedite the development and review of drugs that show substantial improvement over existing therapies. This regulatory environment encourages pharmaceutical companies to invest in the development of immune checkpoint inhibitors, as they can bring their products to market more swiftly. As a result, the number of approved immune checkpoint inhibitors has increased, providing patients with more treatment options and driving market growth in the US.
Increasing Awareness and Education
The US Immune Checkpoint Inhibitors Market is also influenced by increasing awareness and education regarding cancer treatments. Healthcare professionals and patients are becoming more informed about the benefits of immune checkpoint inhibitors, leading to higher adoption rates. Organizations such as the American Society of Clinical Oncology (ASCO) are actively promoting educational initiatives that highlight the importance of these therapies in cancer management. This growing awareness is likely to translate into increased demand for immune checkpoint inhibitors, as patients seek out these innovative treatment options. Consequently, the market is expected to expand as more individuals become aware of the potential benefits of these therapies.
Advancements in Research and Development
The US Immune Checkpoint Inhibitors Market benefits from significant advancements in research and development. Pharmaceutical companies are investing heavily in the discovery of new immune checkpoint inhibitors and combination therapies. The National Institutes of Health (NIH) has reported a substantial increase in funding for cancer research, which has facilitated the development of novel therapies. This influx of resources is likely to accelerate the introduction of innovative treatments into the market, enhancing the competitive landscape. Furthermore, ongoing clinical trials are exploring the efficacy of these inhibitors in various cancer types, which may lead to new approvals and broaden the scope of treatment options available to patients in the US.
Rising Investment in Personalized Medicine
The US Immune Checkpoint Inhibitors Market is significantly impacted by the rising investment in personalized medicine. As the understanding of cancer biology evolves, there is a growing emphasis on tailoring treatments to individual patient profiles. This shift towards personalized medicine is driving the development of targeted therapies, including immune checkpoint inhibitors. The National Cancer Institute has reported an increase in funding for research focused on personalized approaches to cancer treatment. This trend is likely to enhance the effectiveness of immune checkpoint inhibitors, as they can be matched to specific patient populations based on genetic and molecular characteristics. As personalized medicine continues to gain traction, the market for immune checkpoint inhibitors is expected to grow.