Enhanced Patient Support Networks
Enhanced patient support networks are emerging as a vital component in the fibrous dysplasia market. These networks provide resources, education, and emotional support to individuals affected by fibrous dysplasia, fostering a sense of community among patients and their families. Organizations dedicated to rare diseases are increasingly offering platforms for patients to share experiences and access information about treatment options. This support can lead to improved patient adherence to treatment regimens and better health outcomes. As these networks grow, they may also influence healthcare providers to prioritize the needs of fibrous dysplasia patients, ultimately driving demand for specialized care and services within the market.
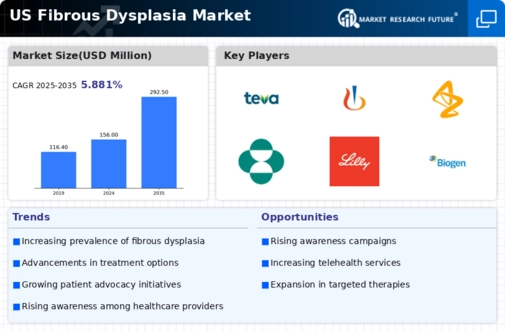
Rising Incidence of Fibrous Dysplasia
The increasing incidence of fibrous dysplasia in the US is a notable driver for the fibrous dysplasia market. Recent estimates suggest that the prevalence of this condition may be around 1 in 100,000 individuals, leading to a growing patient population requiring specialized care. As awareness of this rare bone disorder expands, healthcare providers are more likely to diagnose and treat affected individuals, thereby stimulating demand for therapeutic options. This trend is further supported by advancements in diagnostic imaging techniques, which enhance the identification of fibrous dysplasia cases. Consequently, the fibrous dysplasia market is poised for growth as more patients seek treatment and management solutions.
Innovations in Pharmaceutical Treatments
Innovations in pharmaceutical treatments for fibrous dysplasia are significantly impacting the market landscape. The development of targeted therapies and novel drug formulations is expected to enhance treatment efficacy and patient outcomes. For instance, recent studies have indicated that bisphosphonates and other medications can effectively manage symptoms and complications associated with fibrous dysplasia. As these innovative treatments gain regulatory approval and enter the market, they are likely to attract investment and interest from pharmaceutical companies. This influx of new therapies could potentially increase the overall market size, as healthcare providers and patients seek the most effective options available in the fibrous dysplasia market.
Regulatory Support for Treatment Approvals
Regulatory support for treatment approvals is a significant driver in the fibrous dysplasia market. The US Food and Drug Administration (FDA) has been actively working to expedite the approval process for therapies targeting rare diseases, including fibrous dysplasia. This regulatory environment encourages pharmaceutical companies to invest in research and development, knowing that there is a pathway for bringing new treatments to market more efficiently. As a result, the fibrous dysplasia market may see an influx of innovative therapies that can address the unmet needs of patients. This supportive regulatory framework not only enhances the market's growth potential but also fosters a competitive landscape among companies developing treatments for fibrous dysplasia.
Growing Investment in Rare Disease Research
The growing investment in rare disease research is a crucial driver for the fibrous dysplasia market. With increased funding from both public and private sectors, research initiatives are focusing on understanding the underlying mechanisms of fibrous dysplasia and developing effective treatments. In the US, organizations dedicated to rare diseases are advocating for more resources to be allocated towards research, which may lead to breakthroughs in treatment options. This heightened focus on rare diseases not only raises awareness but also encourages collaboration among researchers, healthcare providers, and pharmaceutical companies. As a result, the fibrous dysplasia market is likely to benefit from enhanced research efforts and the introduction of innovative therapies.