Government Initiatives and Funding
Government initiatives aimed at reducing cervical cancer incidence are significantly influencing the Cervical Dysplasia Market. Various countries have implemented national screening programs and vaccination campaigns against HPV, which are crucial in preventing cervical dysplasia. For instance, funding for cervical cancer prevention programs has seen an increase, with some governments allocating millions to enhance screening accessibility. This financial support not only facilitates the implementation of preventive measures but also raises public awareness about cervical health. As these initiatives gain momentum, the Cervical Dysplasia Market is expected to expand, driven by increased participation in screening programs and the adoption of preventive healthcare practices.
Advancements in Diagnostic Technologies
Technological innovations in diagnostic tools are transforming the Cervical Dysplasia Market. The introduction of advanced screening methods, such as liquid-based cytology and HPV testing, has improved the accuracy and efficiency of cervical cancer detection. These advancements not only enhance patient outcomes but also encourage more women to undergo regular screenings. The market for cervical cancer diagnostics is projected to grow at a compound annual growth rate of around 7% over the next few years, driven by these technological improvements. As healthcare systems increasingly adopt these cutting-edge technologies, the Cervical Dysplasia Market is likely to experience substantial growth, reflecting the demand for more reliable and effective diagnostic solutions.
Growing Incidence of Cervical Dysplasia
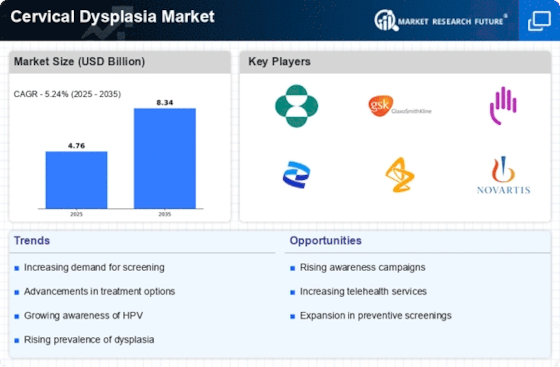
The rising incidence of cervical dysplasia is a critical factor propelling the Cervical Dysplasia Market. Epidemiological studies indicate that the prevalence of cervical dysplasia has been on the rise, particularly among women aged 21 to 29. This trend is largely attributed to the increasing rates of HPV infections, which are known to be the primary cause of cervical dysplasia. As the number of diagnosed cases continues to grow, there is a corresponding increase in the demand for diagnostic and therapeutic services. This surge in incidence is likely to drive investments in research and development within the Cervical Dysplasia Market, as stakeholders seek to develop more effective treatment options and improve patient care.
Increasing Awareness of Cervical Health
The rising awareness regarding cervical health is a pivotal driver for the Cervical Dysplasia Market. Educational campaigns and initiatives by health organizations have significantly contributed to the understanding of cervical dysplasia and its implications. As more women become informed about the risks associated with HPV and cervical cancer, the demand for screening and preventive measures is likely to increase. This heightened awareness is reflected in the growing number of women participating in regular screenings, which has been reported to rise by approximately 20% in recent years. Consequently, this trend is expected to bolster the Cervical Dysplasia Market, as healthcare providers adapt to meet the increasing demand for diagnostic and therapeutic services.
Integration of Preventive Healthcare Practices
The integration of preventive healthcare practices into routine medical care is reshaping the Cervical Dysplasia Market. As healthcare providers increasingly emphasize preventive measures, such as regular screenings and HPV vaccinations, the focus shifts from treatment to prevention. This proactive approach is expected to lead to a decline in the incidence of cervical dysplasia over time, but it also creates a robust market for preventive services. The Cervical Dysplasia Market is likely to benefit from this shift, as more healthcare systems adopt comprehensive screening protocols and vaccination programs. The emphasis on prevention not only enhances patient outcomes but also reduces long-term healthcare costs, making it a compelling driver for market growth.