Enhanced Research Funding
The ebstein s-anomaly market is benefiting from enhanced research funding aimed at understanding congenital heart defects. Government and private organizations are increasingly allocating resources to research initiatives that focus on the etiology, diagnosis, and treatment of these conditions. For instance, the National Institutes of Health (NIH) has dedicated millions of dollars to research on congenital heart diseases, which includes ebstein s-anomaly. This influx of funding not only supports clinical trials and innovative treatment approaches but also fosters collaboration among researchers, healthcare professionals, and pharmaceutical companies. As a result, advancements in treatment modalities and improved patient outcomes are anticipated, further propelling the growth of the ebstein s-anomaly market.
Technological Innovations in Cardiac Care
Technological innovations are playing a pivotal role in shaping the ebstein s-anomaly market. The advent of advanced imaging techniques, such as 3D echocardiography and cardiac MRI, has significantly improved the accuracy of diagnosis and assessment of ebstein s-anomaly. These technologies enable healthcare providers to visualize the heart's structure and function in unprecedented detail, facilitating better treatment planning. Moreover, the development of minimally invasive surgical techniques has transformed the management of this condition, allowing for quicker recovery times and reduced hospital stays. As these technologies continue to evolve, they are likely to enhance the overall quality of care for patients with ebstein s-anomaly, thereby driving market growth.
Growing Patient Advocacy and Support Groups
The ebstein s-anomaly market is also influenced by the growing presence of patient advocacy and support groups. These organizations play a crucial role in raising awareness about ebstein s-anomaly and providing resources for affected individuals and families. By fostering community engagement and education, these groups help to improve the understanding of the condition among both patients and healthcare providers. This increased awareness can lead to earlier diagnosis and intervention, which is essential for better health outcomes. Furthermore, advocacy efforts often result in increased funding for research and improved access to care, thereby positively impacting the ebstein s-anomaly market.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a key driver in the ebstein s-anomaly market. The US Food and Drug Administration (FDA) has implemented various initiatives to expedite the approval process for novel treatments and devices aimed at addressing congenital heart defects. This regulatory environment encourages pharmaceutical companies and medical device manufacturers to invest in research and development for therapies specifically targeting ebstein s-anomaly. As a result, the market is likely to see a surge in the introduction of new treatment options, which could enhance patient care and improve outcomes. The proactive stance of regulatory bodies is expected to foster innovation and growth within the ebstein s-anomaly market.
Increasing Prevalence of Congenital Heart Defects
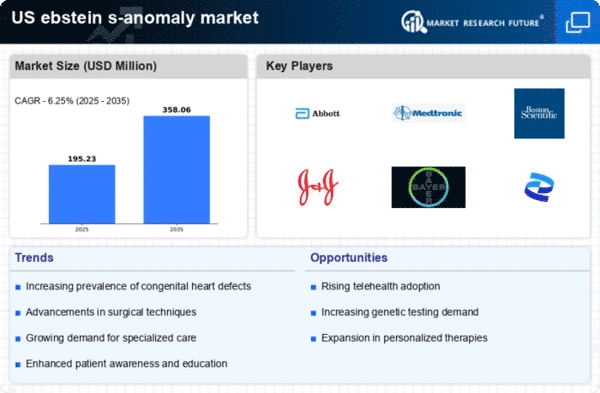
The ebstein s-anomaly market is experiencing growth due to the rising prevalence of congenital heart defects in the US. Recent data indicates that congenital heart defects affect approximately 1 in 100 live births, with ebstein s-anomaly being one of the less common forms. This increase in diagnosed cases leads to a higher demand for specialized treatments and interventions, thereby driving the market. As healthcare providers become more adept at identifying these conditions early, the number of patients requiring ongoing management and care is likely to rise. Consequently, this trend is expected to bolster the ebstein s-anomaly market, as more patients seek tailored therapies and surgical options to address their specific needs.