Technological Innovations in Treatment
Technological advancements in the development of therapies for chorea disorders are significantly influencing the chorea market. Innovations such as gene therapy and targeted drug delivery systems are emerging as potential game-changers in treatment efficacy. For instance, recent breakthroughs in gene editing technologies may offer new avenues for addressing the underlying genetic causes of chorea. The market for neurological therapeutics is projected to reach approximately $100 billion by 2026, highlighting the financial potential of these innovations. Furthermore, the integration of digital health solutions, including telemedicine and mobile health applications, is enhancing patient monitoring and treatment adherence. These technological developments not only improve patient outcomes but also attract investment and interest from pharmaceutical companies, thereby fostering a dynamic environment within the chorea market.
Increased Focus on Patient-Centric Care
The shift towards patient-centric care models is reshaping the landscape of the chorea market. Healthcare providers are increasingly prioritizing the needs and preferences of patients, leading to more personalized treatment approaches. This trend is particularly relevant for chorea disorders, where symptoms can vary widely among individuals. The emphasis on patient engagement and shared decision-making is likely to drive demand for tailored therapies and support services. Furthermore, organizations advocating for patients with chorea are gaining traction, raising awareness and promoting access to care. This focus on patient-centricity may also influence regulatory policies, encouraging the development of treatments that align with patient needs. As a result, the chorea market is expected to evolve in ways that enhance patient satisfaction and improve overall health outcomes.
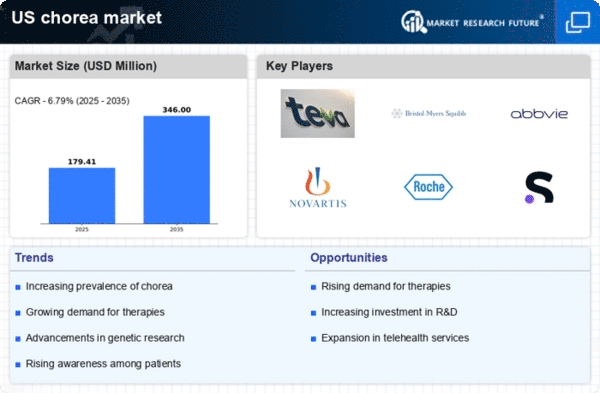
Increasing Prevalence of Chorea Disorders
The rising incidence of chorea disorders in the US is a notable driver for the chorea market. Conditions such as Huntington's disease and Sydenham's chorea are becoming more recognized, leading to a greater demand for diagnostic and therapeutic solutions. Recent estimates suggest that approximately 5-10 individuals per 100,000 are affected by Huntington's disease alone, indicating a substantial patient population. This growing prevalence necessitates enhanced healthcare resources and innovative treatment options, thereby stimulating market growth. As awareness increases, healthcare providers are more likely to invest in advanced diagnostic tools and therapies, further propelling the chorea market. The need for effective management strategies for these disorders is likely to drive research and development efforts, ultimately benefiting patients and healthcare systems alike.
Growing Investment in Neurological Research
The increasing investment in neurological research is a critical driver for the chorea market. Government and private sector funding for studies focused on chorea and related disorders is on the rise, reflecting a commitment to understanding these complex conditions. In 2025, funding for neurological research in the US is expected to exceed $30 billion, with a portion allocated specifically for chorea-related studies. This influx of capital is likely to accelerate the development of new therapies and diagnostic tools, addressing unmet medical needs. Additionally, collaborations between academic institutions and pharmaceutical companies are becoming more common, fostering innovation and expediting the translation of research findings into clinical applications. As a result, the chorea market stands to benefit from enhanced research capabilities and a more robust pipeline of treatment options.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a significant driver impacting the chorea market. The US Food and Drug Administration (FDA) has implemented various initiatives aimed at expediting the approval process for novel treatments, particularly those addressing rare neurological disorders. This regulatory environment encourages pharmaceutical companies to invest in research and development for chorea therapies, as the pathway to market entry becomes more streamlined. In recent years, the FDA has granted breakthrough therapy designations to several promising candidates targeting chorea symptoms, indicating a favorable outlook for new treatments. This supportive regulatory framework not only enhances the attractiveness of the chorea market for investors but also holds the potential to bring much-needed therapies to patients more rapidly. As a result, the market is likely to experience growth driven by the introduction of innovative solutions.